DNA/RNA Extraction of Pentagona and Hystera Sea Stars
Notes
Sample Preparation
- Samples:
- CPAB-003
- CHSB-005
- CPBO-008
- CPOI-009
- CPAB-011
- CPDB-001
- CPBP-001
- CHSY-010
- Thawed samples on ice bucket
- Prepared forceps, foil, and razor blades
- Cleaned forceps with 10% bleach, DI water, 70% ethanol, and RNaseZap before and between each sample
- Made 1 bead tube per sample with 700ul of DNA/RNA shield in each
- Cut each tissue piece in half and placed in the bead tube
- CPAB-003

- CHSB-005

- CPBO-008

- CPOI-009

- CPAB1-011
- CPDB-001
- CPBP-001

- CHSY-010
- Vortexed the bead tubes for 1 minute at max speed
- The tissue didn’t look that broken up but I continued with the protocol that I had tired previously
- Spun down the tubes to remove bubbles (there were still some)
- Removed all the volume I could from the bead tubes into new 1.5mL tubes ~450ul
- Saved the bead tubes by adding 300ul DNA/RNA Shield to each and freezing them. They looked like:
- Added 22.5ul Proteinase K and 45ul Pro K digestion buffer to each 1.5mL tube
- Vortexed and spun down the tubes
- Added equal volume (520ul) of DNA/RNA lysis buffer to each tube
- Flicked and inverted tubes to mix and spun down
- Warmed 10mM Tris HCl pH 8 and nuclease free water in the thermomixer at 70 degrees C
- Added 700ul of the liquid to yellow spin columns and collection tubes
- Centrifuged 16,000rcf for 30 seconds
- Saved the flow through in new 5mL tubes
- Added the remaining liquid to the yellow spin columns
- Centrifuged 16,000rcf for 30 seconds
- Saved the flow through in the 5mL tubes
- Added 400ul DNA/RNA prep buffer to the yellow spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 700ul DNA/RNA wash buffer to the yellow spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 400ul DNA/RNA wash buffer to the yellow spin columns
- Centrifuged 16,000rcf for 2 minutes
- Transferred spin columns to new 1.5mL tubes for each sample labeled as final DNA tubes
- Discarded flow through and collection tubes
- Added 50ul warmed 10mM Tris HCl to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Added another 50ul warmed 10mM Tris HCl to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Discarded the spin columns
- Made strip tubes with 7ul of each sample in them for QC
- Kept tubes on ice bucket then stored in -20 degree freezer
- Added equal volume (1000ul) 100% ethanol to each 5mL tube
- Vortexed and spun down
- Added 700ul of the liquid to green spin columns and collection tubes
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Repeated addition for the remaining liquid (2 times) to the green spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 400ul DNA/RNA wash buffer to the green spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Created the DNase mix:
- 75ul DNA digestion buffer * 8 = 600ul
- 5ul DNase I * 8 = 40ul
- Flicked and spun down mix
- Added 80ul DNAse mix to each green spin column filter
- Incubated for 15 minutes at room temp
- Added 400ul DNA/RNA prep buffer to the green spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 700ul DNA/RNA wash buffer to the green spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 400ul DNA/RNA wash buffer to the green spin columns
- Centrifuged 16,000rcf for 2 minutes
- Transferred spin columns to new 1.5mL tubes for each sample labeled as final RNA tubes
- Discarded flow through and collection tubes
- Added 50ul warmed nuclease free water to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Added another 50ul warmed nuclease free water to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Discarded the spin columns
- Made strip tubes with 5ul of each sample in them for QC
- Kept tubes on ice bucket then stored in -80 degree freezer
QC
Broad Range Qubit for DNA and RNA
| Sample |
Reading 1 (ng/ul) |
Reading 2 (ng/ul) |
Average DNA (ng/ul) |
| Standard 1 |
190 RFU |
- |
- |
| Standard 2 |
21286 RFU |
- |
- |
| CPAB-003 |
62.4 |
61.4 |
61.9 |
| CHSB-005 |
7.18 |
6.96 |
7.07 |
| CPBO-008 |
4.28 |
4.2 |
4.24 |
| CPOI-0009 |
10.7 |
10.6 |
10.65 |
| CPAB1-011 |
19.9 |
19.7 |
19.8 |
| CPDB-001 |
17.5 |
17.4 |
17.45 |
| CPBP-001 |
18.6 |
18.5 |
18.55 |
| CHSY-010 |
12 |
11.9 |
11.95 |
| Sample |
Reading 1 (ng/ul) |
Reading 2 (ng/ul) |
Average RNA (ng/ul) |
| Standard 1 |
408 RFU |
- |
- |
| Standard 2 |
8288 RFU |
- |
- |
| CPAB-003 |
25.2 |
24.8 |
25 |
| CHSB-005 |
too low |
- |
- |
| CHSB-005 |
too low |
- |
- |
| CPBO-008 |
too low |
- |
- |
| CPOI-0009 |
too low |
- |
- |
| CPAB1-011 |
too low |
- |
- |
| CPDB-001 |
too low |
- |
- |
| CPBP-001 |
13.4 |
12.8 |
13.1 |
| CHSY-010 |
too low |
- |
- |
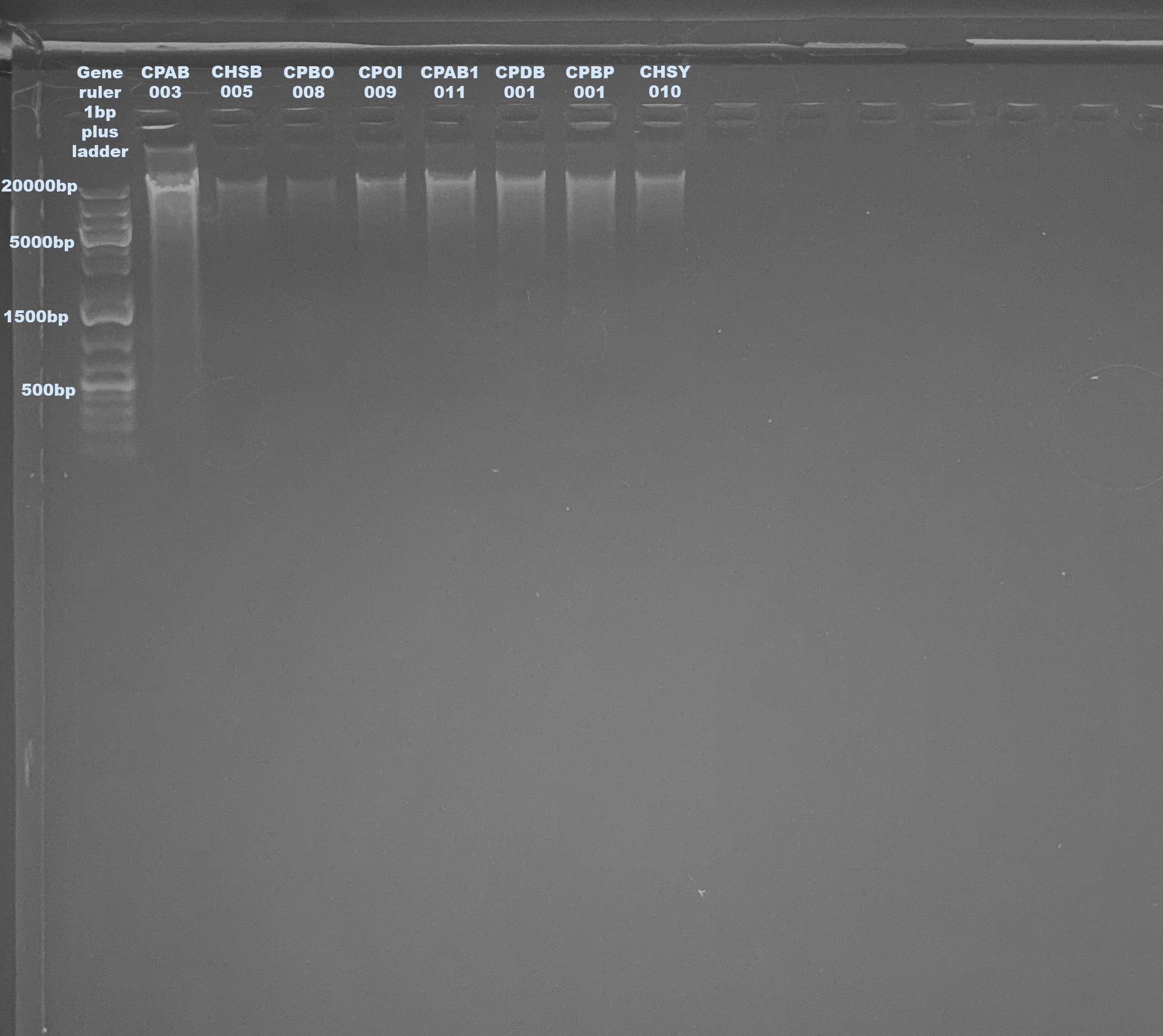
1% Agarose Gel for DNA Quality
RNA TapeStation
Notes
- Quality of DNA and RNA is good but yield is LOW especially of the RNA
- I think the homogenization was not efficient and also the small sample pieces need the most efficient
- Next extraction I will try to chop the pieces before homogenization and maybe add incubation too
Written on October 15, 2020