Qiagen Genomic Tip HMW DNA Extraction of Porites lobata
HMW DNA Extraction for Pac-Bio Sequencing of Porites lobata Flash Frozen Tissue Chunk
In this sample processing I use the QIAGEN Genomic-tip 100/G, the QIAGEN Genomic DNA Buffer Set, QIAGEN RNase A (100mg/mL concentration), QIAGEN Proteinase K, and DNA lo-bind tubes
The sample used was named “Porites 6” and was flash frozen chunks of coral tissue and skeleton sent to use from Hawai’i. The species was determined to be Porites lobata by Sanger sequencing using the ITS region. The primers used for species ID were:
Por ITSZ1 - 5’-TAAAAGTCGTAACAAGGTTTCCGTA-3’
Por ITSZ2 - 5’-CCTCCGCTTATTGATATGCTTAAAT-3’
The sequences analyzed using BLASTn (output here) indicated the closest alignment to Porites lobata. Sanger sequencing on the ITS region and DNA prep was performed by Crawford Drury and Eva Majerová at the Gates Coral Lab at the Hawai’i Institute of Marine Biology.
Goal: Get 25ug of HMW DNA from Porites 6 flash frozen chunks sent from HI
Result: 130ug of DNA! This is so much, most of it is HMW but there is a lot of smearing into the lower bp
Major Take Aways: I think I may have used too much tissue, I was trying to use more because the previous extraction with this method yielded just below what I was aiming for. I may have to do this again to get less smearing of the DNA.
Sample Processing
Set up
- Prepared digestion buffer with 9.5mL of Buffer G2 and 19ul of RNase A
- Set incubator genie to 50 degrees C
- Cleaned forceps, and scraper/scooper with 10% bleach, DI water, then 70% EtOH
- Wrapped those in foil and placed on dry ice
- Also placed mortar and pestle in -80
- Had styrofoam cooler of dry ice saved from a shipment and filled Thermoflask dewar with LN2
- Brought over scale to bench
Grinding and Incubation
- Using sample Porites 6, flash frozen chunks in a 50mL conical
- Picked a small chunk with a good amount of tissue on it and another chip that was mostly polyps and not skeleton
- Tared the scale with the chilled mortar on it
- Put in the chunk and weighed: 1.54g

- Poured LN2 into mortar and let boil off
- Ground chunk until it was a powder. It took a while because of the skeleton present

- Scrapped into a chilled 50mL conical with the scraper
- Poured in the buffer G2 mix
- Vortexed briefly
- Added 500ul of Proteinase K and vortexed again
- Image of liquid before incubation:
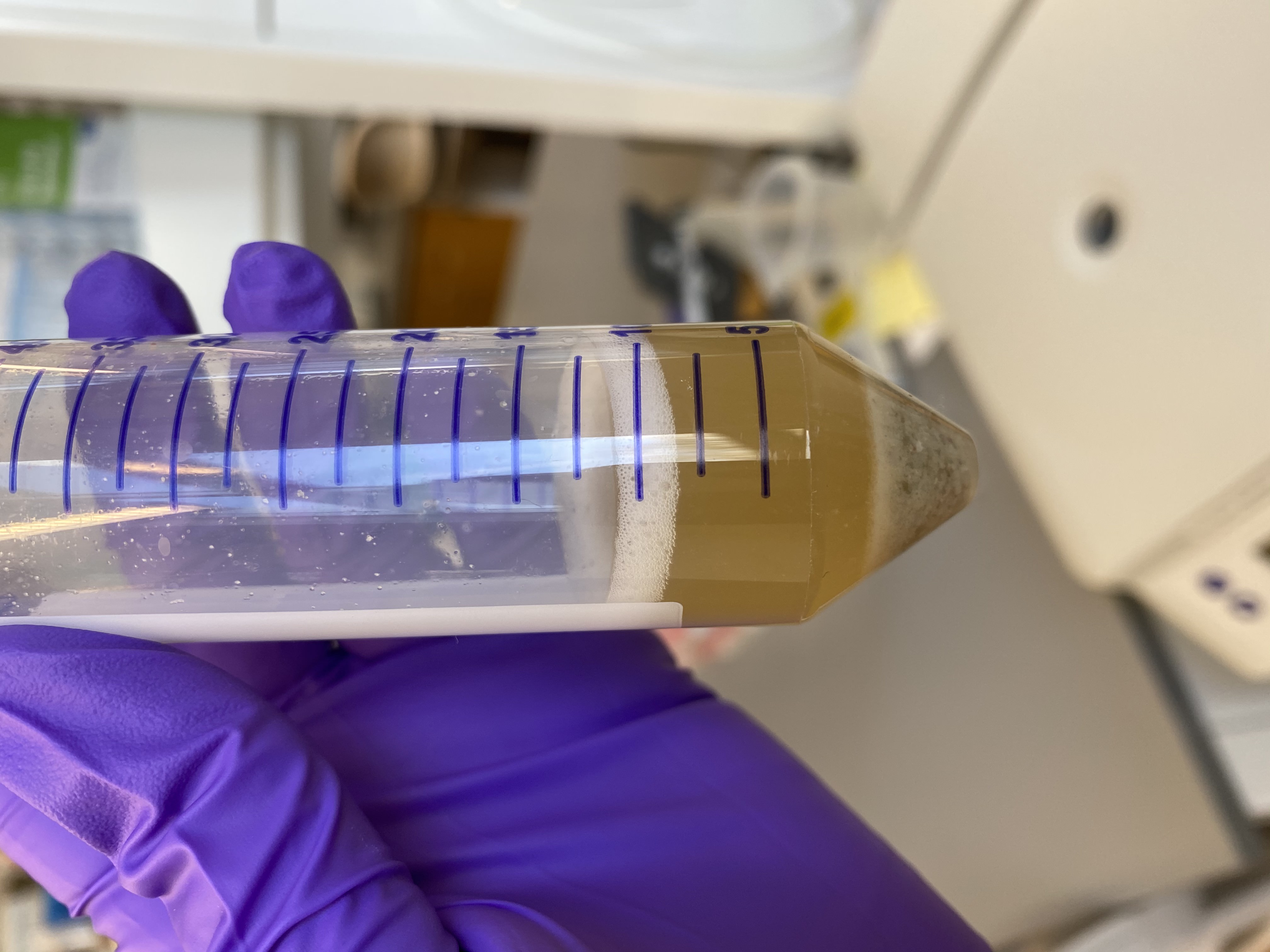
- Put the conical in the incubator genie at 50 degrees C rocking 15 speed for 2 hours
Genomic Tip Extraction
Genomic Tip
- Set centrifuge to 4 degrees C
- Image of liquid after incubation, looks pretty brown and a little opaque but similar to the other extractions with this method, especially the other porites was also very brown:
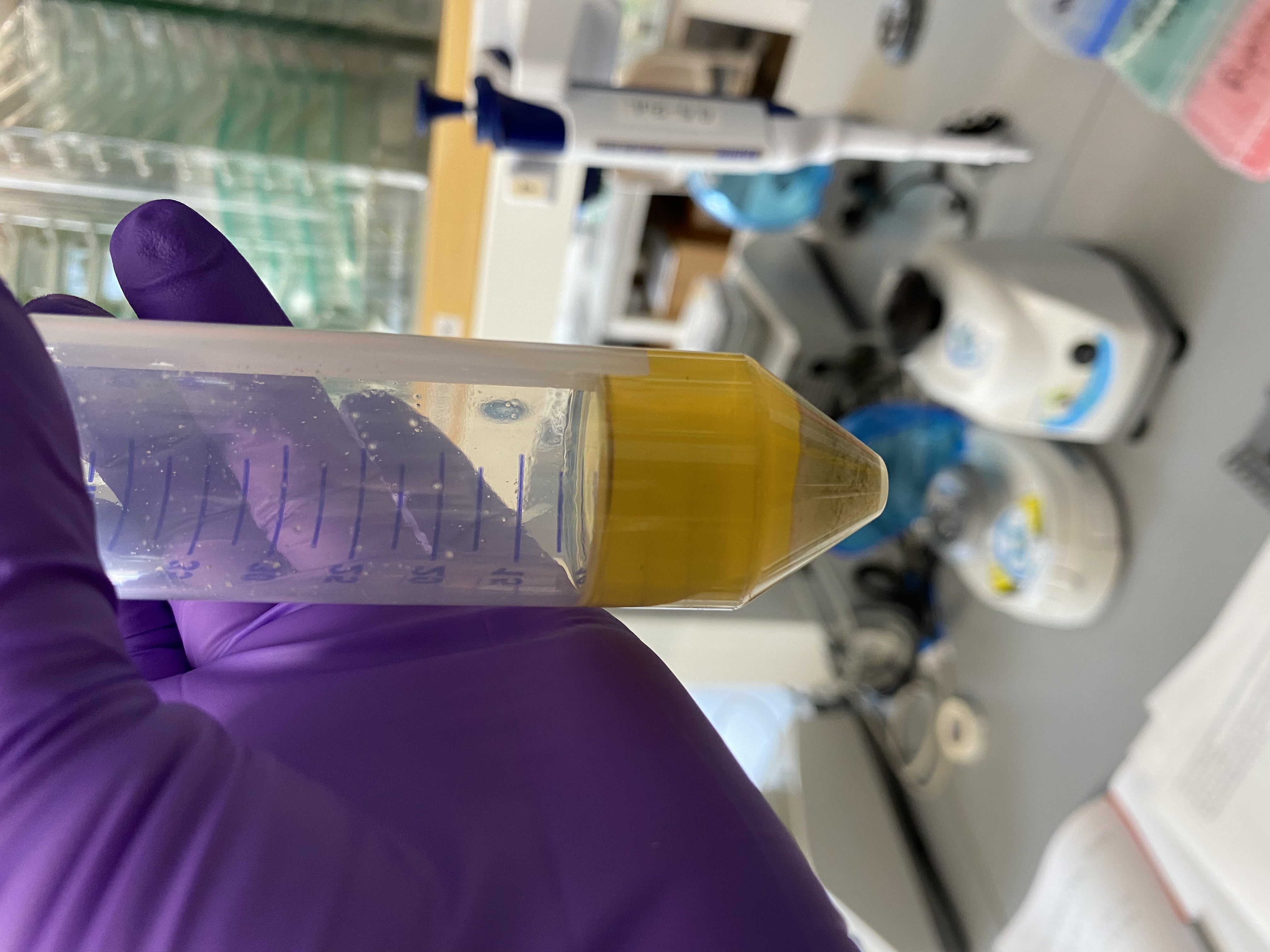
- After incubation, transferred 1mL of lysed tissue liquid into each of 9 1.5mL tubes with wide bore pipette tips
- Centrifuged at 4 degrees C for 10 minutes at 5000 rcf to pellet any extra stuff
- Set up tip (resin column) inside a holder over a 50mL conical
- While that was going, added 4mL of equilibration buffer (QBT) to the tip and let drip through to the conical (took the 10 min)
- After centrifugation sample image, notice there was a sizable brown pellet of debris, the liquid was less opaque as well, however there did end up being some whispy opaque residue at the top of the liquid line I tried to avoid pipetting into the resin tip:

- Added the supernatant from the sample tubes to the resin tip with wide bore pipette tip and let drip through
- Dripping took ~35 minutes (comparable to other Porites extraction)
- Changed 50mL waste conicals
- Added 7.5mL of buffer QC (wash) and let drip through (30min)
- Pipetted 5mL of buffer QF into a 5mL tube and warmed in incubator genie to 50 degrees C
- Repeated wash addition (30 minutes)
- Transferred resin tip to a different 50mL conical
- Added the 5mL of warmed buffer QF and let drip through (20 minutes)
Isopropanol Precipitation of DNA
- Made 7 DNA lo-bind 1.5mL tubes, 6 each with 833ul of the elution liquid, the last tube was about 300ul
- Added 583ul (0.7 volumes) of room temp 100% isopropanol to each of the first 6 tubes and 210ul to the 6th tube
- Gently inverted to mix
- Centrifuged all lo-bind tubes at 10,000 rcf for 30 minutes at 1 degree C
- Made fresh 70% EtOH and placed in -20 freezer to cool down
- Once finished, looked for pellets: there was a visible brown pellet in each tube (note that the liquid going into the tubes before centrifugation was completely clear):

- One tube at a time, removed most of the supernatant
- One tube at a time, added 1mL of cold 70% EtOH and vortexed briefly
- Centrifuged tubes for 10 minutes at 4 degrees C 10,000 rcf
- Removed all of supernatant when finished, used a p200 to get small volumes out
- Let tubes air dry ~7 minutes
- Added 50ul of TE buffer to each of the 7 tubes very gently
- Incubated for 1hr at 55 degrees C in the Theremomixer no shaking
- Once done, transferred to an orbital shaker overnight 300rpm at room temp
QC 20201204
Qubit
- Broad Range Qubit next day (protocol)
- Flicked tube and took from both top T and bottom B of the tube measurements
| Standard 1 | Standard 2 | Sample | Average DNA ng/µl | Averaged top and bottom ng/ul |
|---|---|---|---|---|
| 179 RFU | 20822 RFU | 1 T | 433 | |
| - | - | 1 B | 430 | 431.5 |
| - | - | 2 T | 350 | |
| - | - | 2 B | 337 | 343.5 |
| - | - | 3 T | 450 | |
| - | - | 3 B | 441 | 445.5 |
| - | - | 4 T | 440 | |
| - | - | 4 B | 479 | 459.5 |
| - | - | 5 T | 421 | |
| - | - | 5 B | 448 | 434.5 |
| - | - | 6 T | 484 | |
| - | - | 6 B | 512 | 498 |
| - | - | 7 T | 164.5 | |
| - | - | 7 B | 173 | 168.75 |
Genomic DNA TapeStation
- Followed Tapestation protocol
- Results link
- The tapestation didn’t recognize the largest band in the ladder, I’ve added it in where it should be approximately. However this means it doesn’t size the samples correctly. Additionally all the samples were more higher concentration than it can handle. However, it still looks like there is mostly very HMW DNA in these extractions, with a prominent smear to lower bp, probably due to the large ammout of DNA.
Total Amount of DNA from this extraction based on average top and bottom Qubit values and ~47ul of sample volume in each tube
- 1: 20280.5ng DNA
- 2: 16144.5ng DNA
- 3: 20938.5ng DNA
- 4: 21596.5ng DNA
- 5: 20421.5ng DNA
- 6: 23406.0ng DNA
- 7: 7931.3ng DNA
- TOTAL: 130718.8ng DNA or 130.7ug of DNA