Qiagen Genomic Tip HMW DNA Extraction of Pocillopora meandrina
HMW DNA Extraction for Pac-Bio Sequencing of Pocillopora meandrina Flash Frozen Tissue Chunk
In this process I use the QIAGEN Genomic-tip 100/G, the QIAGEN Genomic DNA Buffer Set, QIAGEN RNase A (100mg/mL concentration), QIAGEN Proteinase K, and DNA lo-bind tubes
Goal: Get 25ug of HMW DNA from this sample
Result: 19ug of DNA, mostly good quality, some smearing present
Major Take Aways: It was very hard to chip this coral, I ended up using a whole chunk, which was very hard to grind. This ended up probably not being enough tissue to get 25ug.
Sample Processing
Set up
- Prepared digestion buffer with 9.5mL of Buffer G2 and 19ul of RNase A
- Set incubator genie to 50 degrees C
- Cleaned forceps, scraper/scooper, and clippers with 10% bleach, DI water, then 70% EtOH
- Wrapped those in foil and placed in -80
- Also placed mortar and pestle in -80
- Had styrofoam cooler of dry ice saved from a shipment and filled Thermoflask dewar with LN2
- Brought over scale
- Set up dry ice box with plastic tube box rack over the ice to have a hard surface for chipping, then covered that in foil
Grinding and Incubation
- Took out sample chunk from conical into dry ice foil and tried to clip with cuticle clippers off just the tissue. The tissue wouldn’t come off very well and the skeleton wouldn’t break
- Tried hammering on the forceps like a chisel, the chunk didn’t split
- After a while I decided that I would never be able to get enough tissue off the chunks like this so I decided to use a whole chunk
- Picked the smallest chunk to try grinding the whole thing
- Tared the scale with the chilled mortar on it
- Put in the chunk and weighed: 1.09g

- Poured LN2 into mortar and let boil off
- Ground chunk until it was a powder. It was very hard to grind as the skeleton is very dense, I used LN2 two more times and really smashed the frozen tissue

- Scrapped into a chilled 50mL conical with the scraper
- Poured in the buffer G2 mix
- Vortexed briefly
- Added 500ul of Proteinase K and vortexed again
- Image of liquid before incubation:

- Put the conical in the incubator genie at 50 degrees C rocking 15 speed for 2 hours
Genomic Tip Extraction
Genomic Tip
- Set centrifuge to 4 degrees C
- Image of liquid after incubation:
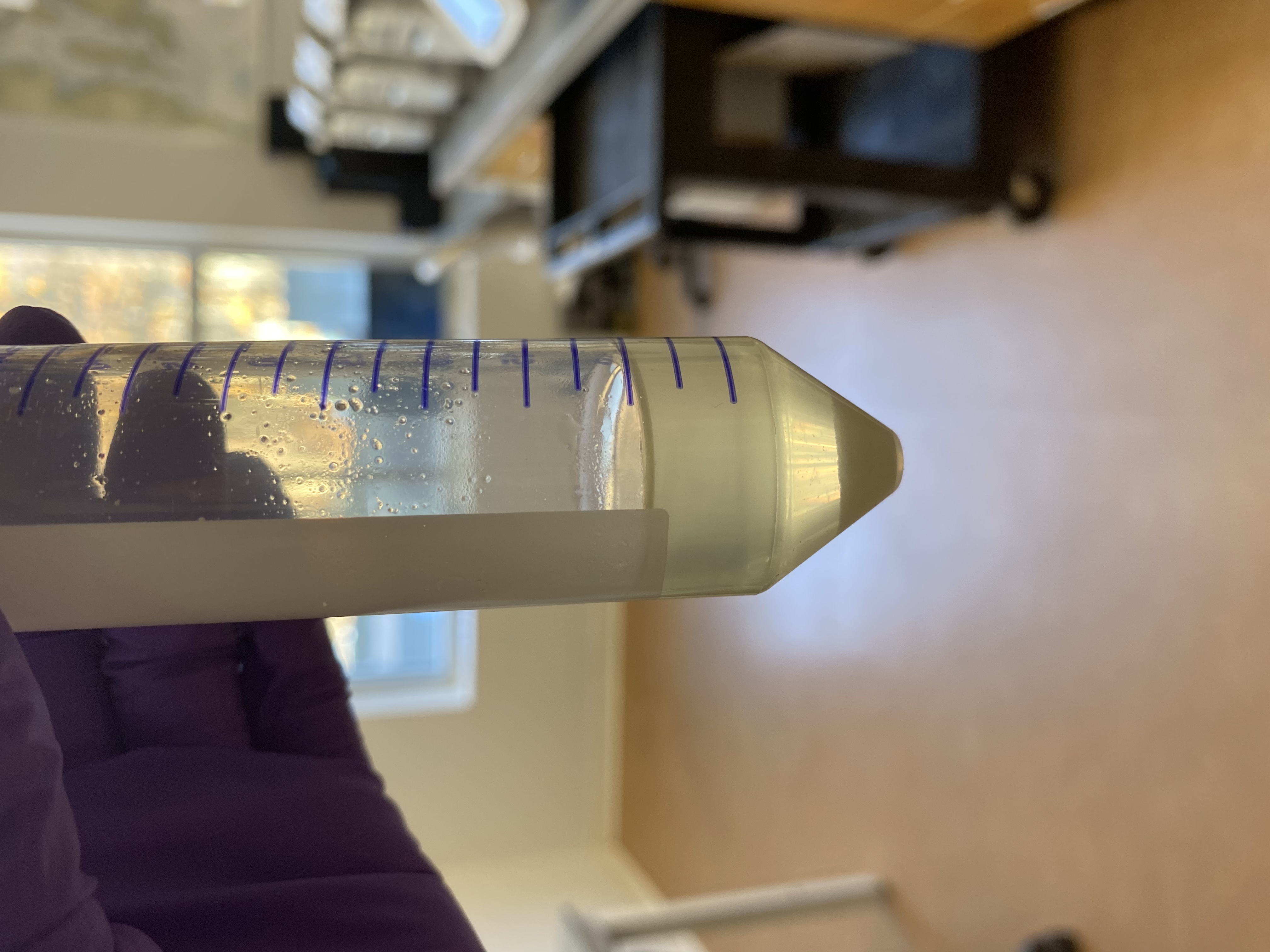
- After incubation, transferred 1mL of lysed tissue liquid into each of 9 1.5mL tubes with wide bore pipette tips
- Centrifuged at 4 degrees C for 10 minutes at 5000 rcf to pellet any extra stuff
- Set up tip (resin column) inside a holder over a 50mL conical
- While that was going, added 4mL of equilibration buffer (QBT) to the tip and let drip through to the conical (took the 10 min)
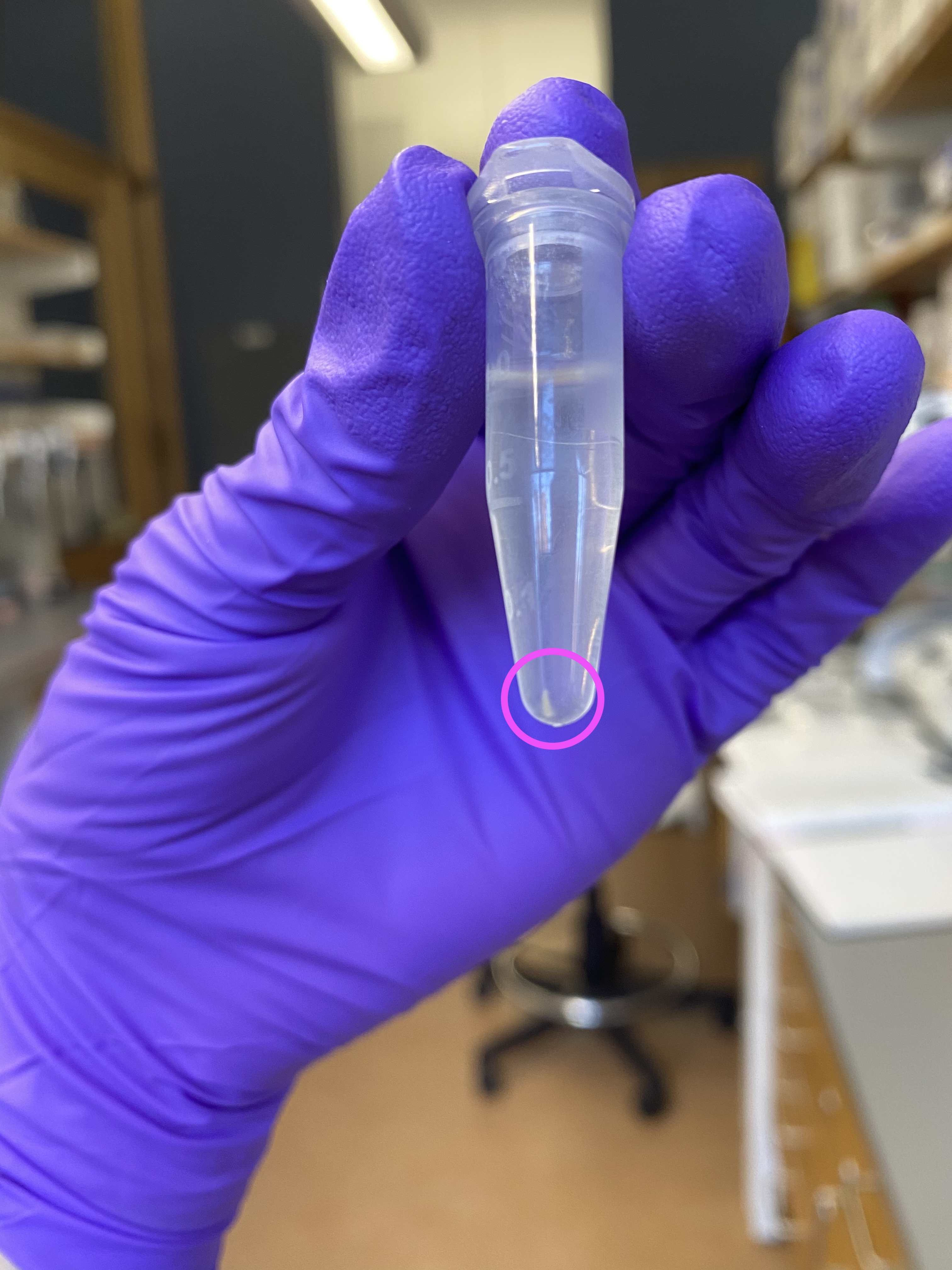
- After centrifugation sample image, notice there was a small white pellet of debris:
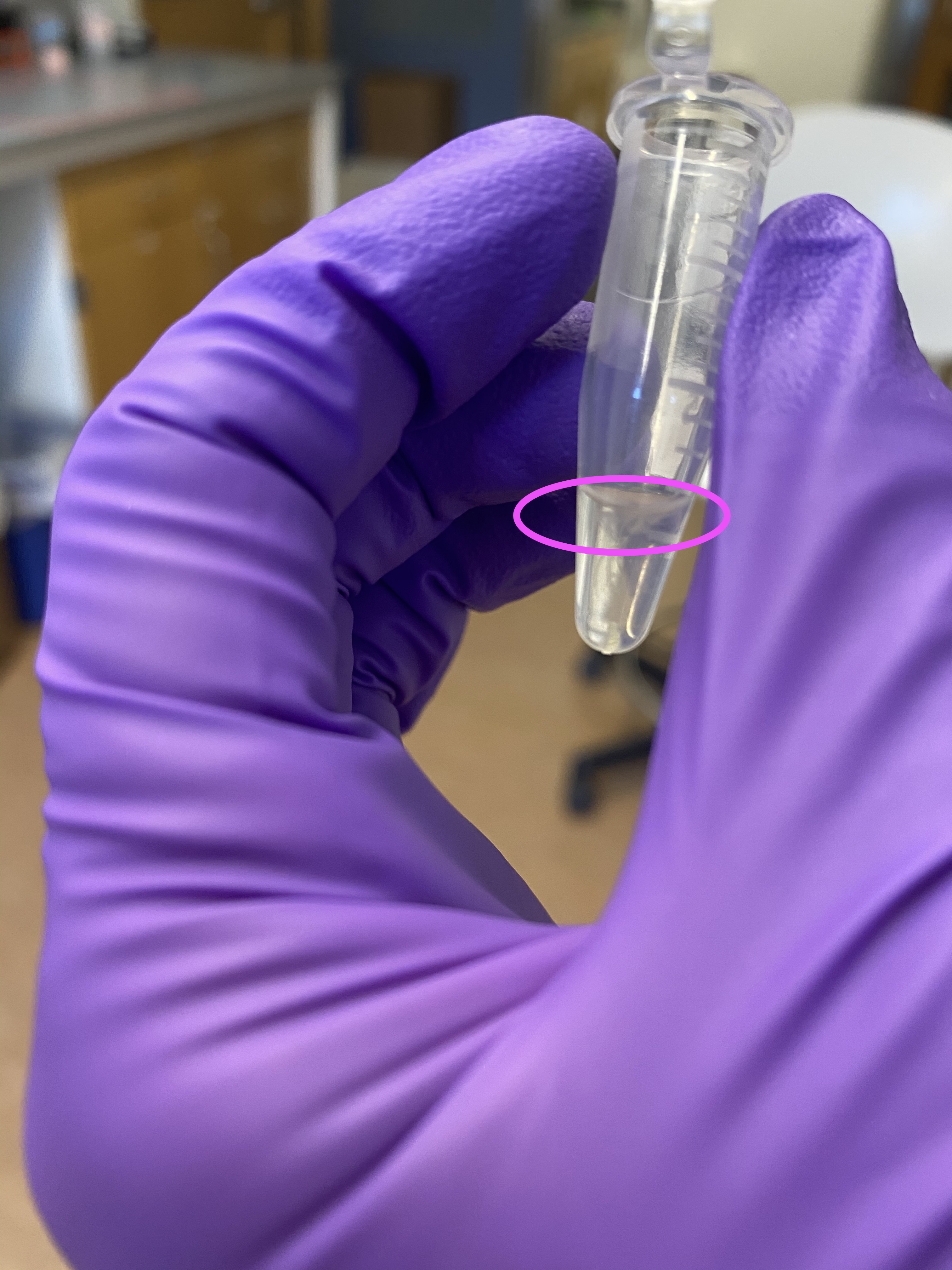
- Also noticed while pipetting out, there was opaque residue at the top of the liquid line, it was hard to avoid pipetting and I wasn’t sure I should keep it or not. I did end up pipetting it but it did not seem to affect dripping rate:
- Added the supernatant from the sample tubes to the resin tip with wide bore pipette tip and let drip through
- Dripping took ~20
- Changed 50mL waste conicals
- Added 7.5mL of buffer QC (wash) and let drip through (10min)
- Pipetted 5mL of buffer QF into a 5mL tube and warmed in incubator genie to 50 degrees C
- Repeated wash addition (10 minutes)
- Transferred resin tip to a different 50mL conical
- Added the 5mL of warmed buffer QF and let drip through (10 minutes)
Isopropanol Precipitation of DNA
- Made 6 DNA lo-bind 1.5mL tubes, 5 each with 833ul of the elution liquid, the last tube was about 700ul
- Added 583ul (0.7 volumes) of room temp 100% isopropanol to each of the first 5 tubes and 490ul to the 6th tube
- Gently inverted to mix
- Centrifuged all lo-bind tubes at 10,000 rcf for 30 minutes at 1 degree C
- Made fresh 70% EtOH and placed in -20 freezer to cool down
- Once finished, looked for pellets: there was a barely visible white-ish pellet in each tube:

- One tube at a time, removed most of the supernatant
- One tube at a time, added 1mL of cold 70% EtOH and vortexed briefly
- Centrifuged tubes for 10 minutes at 4 degrees C 10,000 rcf
- Removed all of supernatant when finished, used a p200 to get small volumes out
- Let tubes air dry ~7 minutes
- Added 50ul of TE buffer to each of the 6 tubes very gently
- Incubated for 1hr at 55 degrees C in the Theremomixer
- Once done, transferred to a shaker overnight 300rpm
QC
Qubit
- Broad Range Qubit next day (protocol)
- Flicked tube and took from both top T and bottom B of the tube measurements
| Standard 1 | Standard 2 | Sample | Average DNA ng/µl | Averaged top and bottom ng/ul |
|---|---|---|---|---|
| 182 RFU | 20306 RFU | 1 T | 65.5 | |
| - | - | 1 B | 73.7 | 69.6 |
| - | - | 2 T | 72.7 | |
| - | - | 2 B | 74.9 | 73.8 |
| - | - | 3 T | 70 | |
| - | - | 3 B | 70.6 | 70.3 |
| - | - | 4 T | 71.4 | |
| - | - | 4 B | 76.8 | 74.1 |
| - | - | 5 T | 60.6 | |
| - | - | 5 B | 66.9 | 63.75 |
| - | - | 6 T | 51 | |
| - | - | 6 B | 58.5 | 54.75 |
Genomic DNA TapeStation
- Followed Tapestation protocol
- Results link
- Most of the DNA is HMW, there is some smearing though
Total Amount of DNA from this extraction based on average top and bottom Qubit values and ~47ul of sample volume in each tube
- 1: 3271.2ng DNA
- 2: 3468.6ng DNA
- 3: 3304.1ng DNA
- 4: 3482.7ng DNA
- 5: 2996.25ng DNA
- 6: 2573.25ng DNA
- TOTAL: 19096.1ng DNA or 19ug of DNA
Written on November 10, 2020