Zymo-Seq RiboFree Total RNA Library Prep Kit Test
Testing RNA Library Prep Kit on Porites Samples for Free Sequencing, using the Zymo-Seq RiboFree Total RNA Library Prep Kit
Using extracted RNA from samples in the previous post, all Porites astreoides
Kit input is 100ng-1µg in a volume of 8µl. Our concentrations of RNA are low, so we are using 125ng of RNA for each sample
Note: All labwork when possible was done at the RNA bench and with filter tips
First Strand cDNA Synthesis
- Thaw samples and reagents R1 and R2 on ice (reagents are kept in the -80)
- Prepare samples to the right volume in PCR strip tubes:
| Sample | volume of sample (125ng) | volume of ultra pure water |
|---|---|---|
| G1-4 | 4.7µl | 3.3µl |
| G1-13 | 4.6µl | 3.4µl |
| Hot #3 1/2 salinity | 6.7µl | 1.3µl |
| TA Amb 1 | 4µl | 4µl |
| TA Amb 2 | 4µl | 4µl |
| TA Hot 2 | 8.1µl | NA |
- Added 2µl reagent R1 to each tubem, pipetted to mix and spun down
- Placed in thermocycler MES login and program ZRibo 1st Strand program”
- 98°C 3 minutes
- 4°C hold
- 25°C 5 minutes
- 48°C 15 minutes
- 4°C hold
- At the first 4°C hold, added 10µl R2 reagent tp each sample in the thermocycler and pipetted to mix
- Pressed enter in the program to continue it
- Took out reagents D1, D2, and D3 to thaw on ice
RiboFree Universal Depletion
- Added 10µl of reagent D1 to each sample on ice, pipetted to mix and spun down
- Placed tubes in thermocycler MES login ZRibo Depletion program:
- 98°C 3 minutes
- 68°C 5 minutes
- 68°C hold
- 68°C 2 hours this is variable in the protocol, I chose 2 hours because it is for over 100ng and below 250ng input
- 68°C hold
- 98°C 2 minutes
- 25°C hold
- At first hold in the thermocyler program added 10µl D2 reagent in the thermocyler without removing the tubes and pipetted to mix
- Pressed enter in the program to continue it
- At the second hold in the thermocycler program added 10µl of the D3 stop solution to each tube in the thermocyler without removing the tubes and pipetted to mix
- Pressed enter in the program to continue it
- Made a 1.5mL tube of 95% EtOH with ultra pure water
- Once the program was done added 25µl of 95% EtOH to each sample, pipetted to mix, and spun down
Cleanup with DNA MagBeads
- Added 24mL of 100% EtOH to the concentrated wash buffer before starting
- Added 1mL of MagBead buffer to the bead concentrate, it was very hard to pipette the concentrated beads, which is why I did not add the beads to the buffer as it says in the protocol note: also the bead concentrate had not shipped upright, so some crusted on the sides of the tube they were in and were not able to be resuspended properly no matter how much I tried, this goes for the other two bead concentrate tubes used later on as well
- Added 150µl MagBead mix to each sample and pipetted at least 10 times each until completely homogenous
- Placed on shaker for 5 minutes
- Placed on magnet plate, and because it was going slow to the magnet (large volume) the plate was placed on the shaker to help ~5-10 min
- Once the supernatant was clear, the clear liquid was removed from each sample without disturbing the beads while still on the magnet plate
- Still on the magnet, added 200µl of Zymo-Seq Wash buffer to each sample
- Removed 200µl of supernatant from each tube without disturbing the beads while still on the magnet
- Repeated: Still on the magnet, added 200µl of Zymo-Seq Wash buffer to each sample
- Removed 200µl of supernatant from each tube without disturbing the beads while still on the magnet
- Used p20 to remove any remaining liquid in the tubes, and used tips to remove any blobs of wash seen left inside each tube
- Resuspended beads in 10µl DNA elution buffer, and placed in a thermocyler already set at 95°C for 5 minutes
- After incubation, placed tubes back on the magnet plate and removed he ~10µl of supernatant into newly labeled PCR tubes
- Placed in 4 degree fridge overnight as a safe stopping point
P7 Adapter Ligation
- Thawed reagents L1 and L2 on ice
- Added 10µl of reagent L1 to each tube from yesterday on ice, pipetted to mix, and spun down
- Placed in thermocyler MES login program ZRibo Adapter 1 program:
- 37°C 15 minutes
- 95°C 2 minutes
- 4°C hold
- 95°C 10 min
- 63°C 30 seconds
- 72°C 7 minutes
- 4°C hold
- At hold in program added 20µl reagent L2 to each tube in the thermocycler, pipetted to mix, and spun down
- Pressed enter in the program to continue it
Cleanup with DNA MagBeads
- Added MagBead Buffer to bead concentrate as above
- After program was over, added 60µl MagBeads to each sample and pipetted at least 10 times each until completely homogenous
- Placed on shaker for 5 minutes
- Placed on magnet plate and placed on the shaker to help
- Once the supernatant was clear, the clear liquid was removed from each sample without disturbing the beads while still on the magnet plate
- Still on the magnet, added 150µl of Zymo-Seq Wash buffer to each sample
- Removed 150µl of supernatant from each tube without disturbing the beads while still on the magnet
- Repeated: Still on the magnet, added 150µl of Zymo-Seq Wash buffer to each sample
- Removed 150µl of supernatant from each tube without disturbing the beads while still on the magnet
- Used p20 to remove any remaining liquid in the tubes, and used tips to remove any blobs of wash seen left inside each tube
- Resuspended beads in 10µl DNA Elution Buffer and placed tubes on the shaker for 5 minutes room temp
- After incubation, placed tubes back on the magnet plate and removed he ~10µl of supernatant into newly labeled PCR tubes
P5 Adapter Ligation
- Thawed reagent L3 on ice
- Added 10µl reagent L3 to each sample on ice, pipetted to mix, and spun tubes down
- Placed in thermocyler MES login ZRibo Adapter 2 program:
- 25°C 15 minutes
- 4°C hold
- After program, brought the volume up in each sample tube to 100µl by adding 80µl DNA elution buffer to each tube
Cleanup with DNA MagBeads
- After program was over, added 100µl MagBeads to each sample and pipetted at least 10 times each until completely homogenous
- Placed on shaker for 5 minutes
- Placed on magnet plate and placed on the shaker to help
- Once the supernatant was clear, the clear liquid was removed from each sample without disturbing the beads while still on the magnet plate
- Still on the magnet, added 200µl of Zymo-Seq Wash buffer to each sample
- Removed 200µl of supernatant from each tube without disturbing the beads while still on the magnet
- Repeated: Still on the magnet, added 200µl of Zymo-Seq Wash buffer to each sample
- Removed 200µl of supernatant from each tube without disturbing the beads while still on the magnet
- Used p20 to remove any remaining liquid in the tubes, and used tips to remove any blobs of wash seen left inside each tube
- Resuspended beads in 20µl DNA Elution Buffer and placed tubes on the shaker for 5 minutes room temp
- After incubation, placed tubes back on the magnet plate and removed he ~20µl of supernatant into newly labeled PCR tubes
Library Index PCR
- Thawed 6 index primers included with the kit (Primer Set L) and the ZymoTaq Premix. Primers have one universal side and one unique side already mixed, at 2.5µM each
- Added 5µl of index primers to each sample tube on ice, making sure each tube got a unique primer:
| Sample | Primer Pair |
|---|---|
| G1-4 | CD Index #77 |
| G1-13 | CD Index #78 |
| Hot #3 1/2 Salinity | CD Index #85 |
| TA Amb 1 | CD Index #86 |
| TA Amb 2 | CD Index #93 |
| TA Hot 2 | CD Index #94 |
- Added 25µl ZymoTaq Premix to each sample tube on ice, pipetted to mix, and spun down
- Placed in thermocycler MES login program ZRibo Index Amp program:
- 95°C 10 minutes
- 95°C 30 seconds (12)
- 60°C 30 seconds (12)
- 72°C 1 minute (12)
- 72°C 7 minutes
- 4 hold
bold steps were cycled through 12 times
- After the PCR, increased the volume in each tube to 100µl by add ing 50µl DNA elution buffer
Cleanup with DNA MagBeads
- Added MagBead Buffer to bead concentrate as above
- After program was over, added 685µl MagBeads to each sample and pipetted at least 10 times each until completely homogenous
- Placed on shaker for 5 minutes
- Placed on magnet plate and placed on the shaker to help
- Once the supernatant was clear, the clear liquid was removed from each sample without disturbing the beads while still on the magnet plate
- Still on the magnet, added 200µl of Zymo-Seq Wash buffer to each sample
- Removed 200µl of supernatant from each tube without disturbing the beads while still on the magnet
- Repeated: Still on the magnet, added 200µl of Zymo-Seq Wash buffer to each sample
- Removed 200µl of supernatant from each tube without disturbing the beads while still on the magnet
- Used p20 to remove any remaining liquid in the tubes, and used tips to remove any blobs of wash seen left inside each tube
- Resuspended beads in 18µl DNA Elution Buffer and placed tubes on the shaker for 5 minutes room temp
- After incubation, placed tubes back on the magnet plate and removed he ~18µl of supernatant into newly labeled PCR tubes
QC
Broad Range DNA Qubit following protocol
| Standard 1 | Standard 2 | Sample | Average DNA ng/µl |
|---|---|---|---|
| 135 RFU | 17106 RFU | G1-4 | 9 |
| - | - | G1-13 | 10.8 |
| - | - | Hot #3 1/3 Salinity | 11.1 |
| - | - | TA Amb 1 | 2.02 |
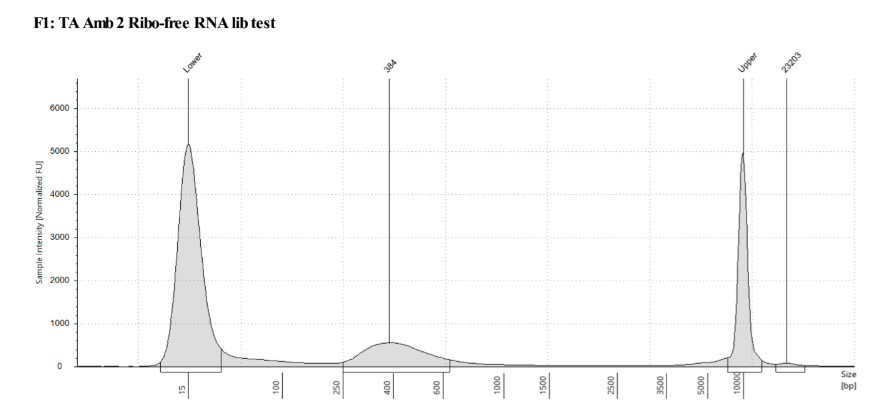
| - | - | TA Amb 2 | 2.5 |
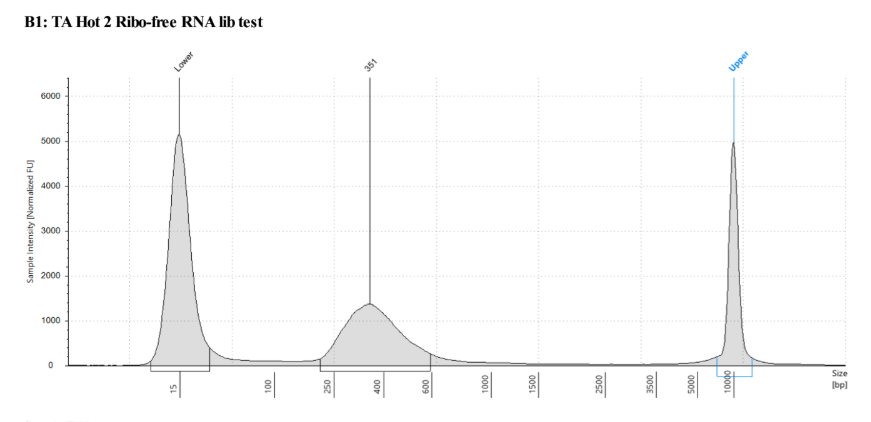
| - | - | TA Hot 2 | 5.36 |
D5000 TapeStation following protocol
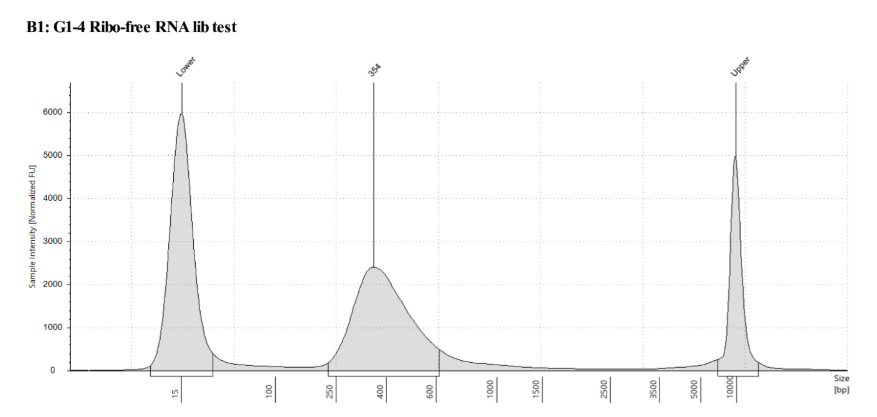
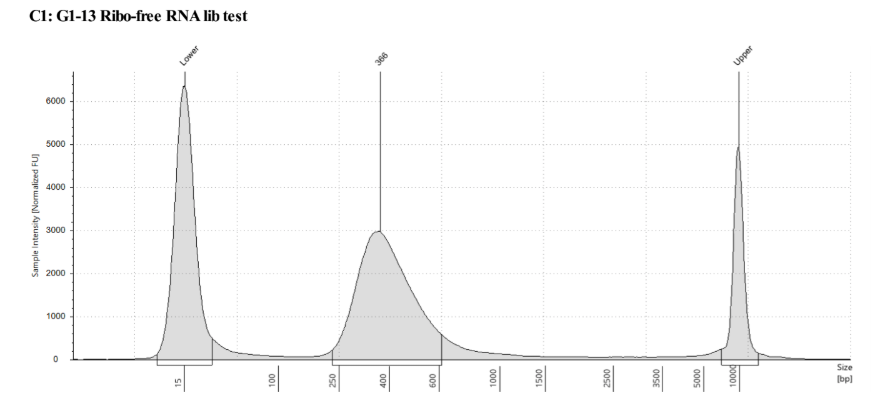
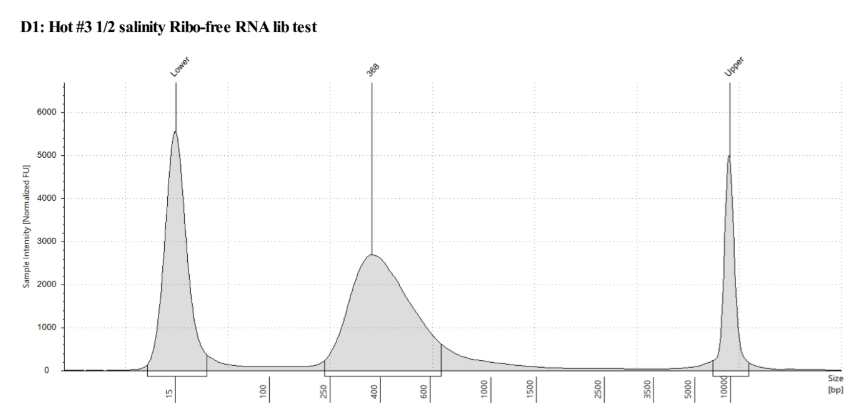
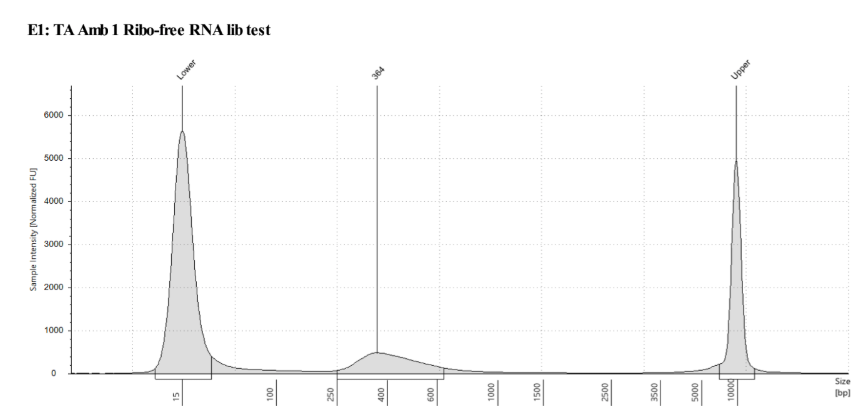
Written on September 5, 2019