Montipora Larvae DNA/RNA Extraction 6
Using Larvae Extraction Protocol on 3 of Ariana’s Montipora Coral Recruits on Flash Frozen Plugs
Goal: Use optimized extraction method on 3 samples for Ariana’s project and practice scraping recruits off of plugs and see if there is enough material for it to work
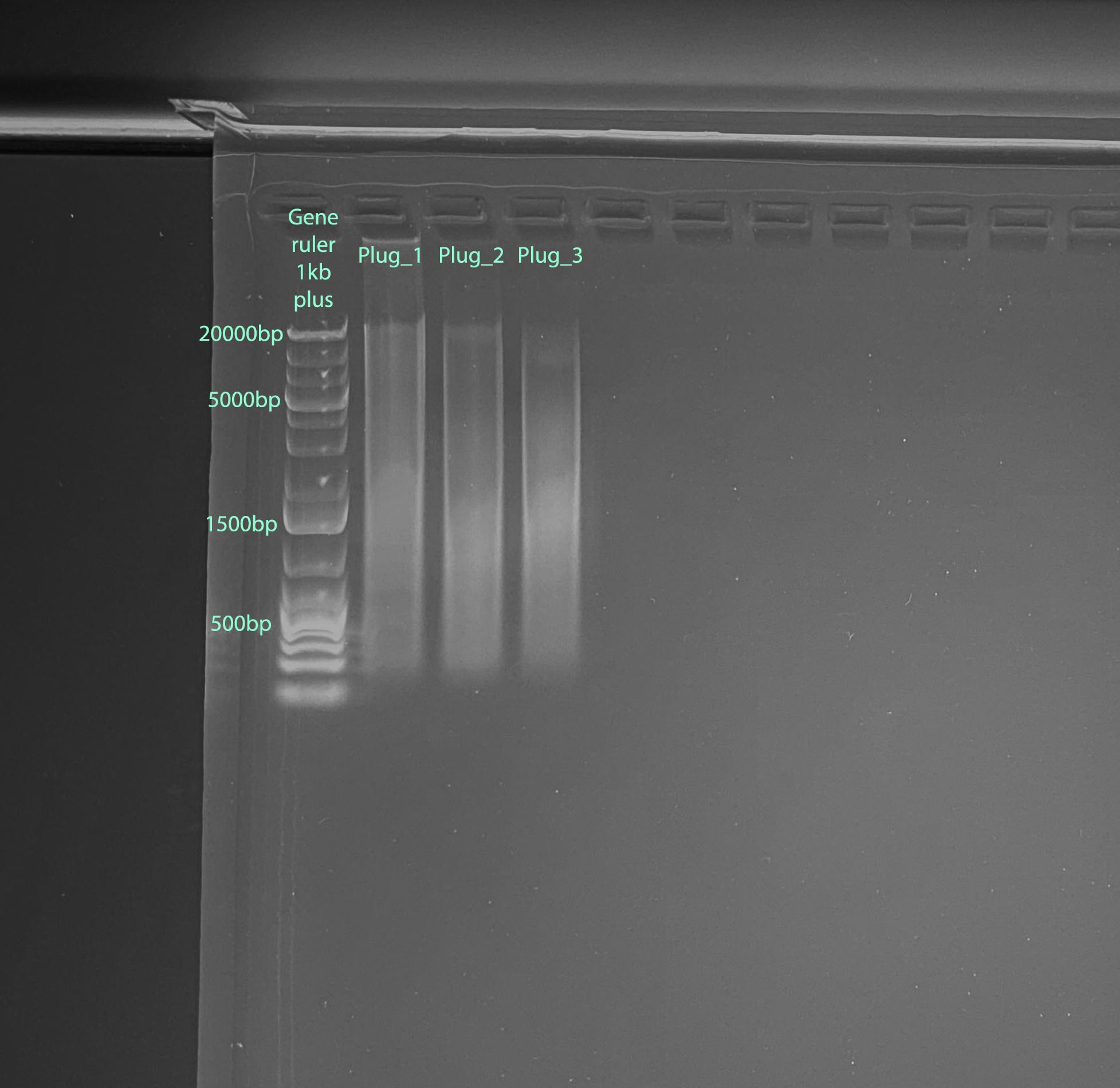
Results: Good DNA and RNA quantity, RNA quality is not very good, and DNA quality is pretty smeared
Take aways: RNA quality and purity is not great, but use-able. The recruits had to thaw pretty much completely to be scrapped off. That may have been the issue. DNA quality is poor, likely because the samples did not need to be homogenized. I am hoping if I don’t use the beads for the other samples it won’t be an issue
Sample Info
Note, the whirl packs didn’t have numbers so I gave them IDs, but when looking at the plugs some had a number on them
| Sample | Timepoint | Number |
|---|---|---|
| Plug_1 | 48hps | 348 |
| Plug_2 | 72hps | – |
| Plug_3 | 5dps | 643 |
I did not save any volume from these because I was worried I didn’t have enough material for the extraction (see pictures below). I also decided to elute all the RNA in 70ul ultrapure water.
Sample Scraping and Homogenizing
- Set up hard snap 1.5mL tubes (the ones that are individually packaged) with 500ul DNA/RNA Shield in each
- Cleaned 3 razor blades with 10% beach, DI water, 70% Ethanol, and RNaseZap in that order and placed on a paper towel
- Took out the first plug and chipped off some ice. Plug_1

- Used a blade to scrape off the brown bits (recruits) onto the blade and scraped them off onto the lip of the 1.5mL tube with the shield. The plug and the recruits sort of melted while I was doing this. I forgot to take a picture of the tube before vortexing it, but for the others I did. For the other plugs I scraped in the same way.
- Plug_1 after scraping:

- I closed the lid of the tube and vortexed it a little to submerge the tissue
- Plug_2 before scraping:

- Plug_2 tube:

- This time I tried using a p1000 pipette tip to push some of the tissue down into the liquid before closing the lid because it kinda gets stuck up there. Then I vortexed it
- Plug_3 before scraping:

- Plug_3 after scraping:

- Plug_3 tube:

- I used the pipette for Plug_3 as well before vortexing
- All three tubes after a brief vortex. The liquid is colored so there is a reasonable amount of tissue, but there aren’t actually any tissue chunks

- I planned to homogenize the same way as the usual protocol: I added half a bead tube to each sample and vortexed for 2 minutes
- Afterwards I centrifuged the bubbles away for 2 min
- Tubes after bead vortexing:

Extraction
- Extraction protocol followed exactly
- Extraction proceeded with ~500ul of each sample
- 500ul of DNA/RNA lysis buffer was used for each sample
- 1mL of 100% ethanol was used for each sample
- All samples were eluted for RNA with 40ul ultrapure water in the first elution and 30ul in the second
QC
Qubit
- Broad Range dsDNA and High Sensitivity RNA Qubit protocol
- All samples were read twice
BR DNA:
| Sample | Standard 1 | Standard 2 | DNA 1 ng/ul | DNA 2 ng/ul | Average ng/ul |
|---|---|---|---|---|---|
| Plug_1 | 177 | 19162 | 37.6 | 36.6 | 37.2 |
| Plug_2 | - | - | 26.4 | 26.6 | 26.5 |
| Plug_3 | - | - | 21.2 | 21 | 21.1 |
HS RNA:
| Sample | Standard 1 | Standard 2 | RNA 1 ng/ul | RNA 2 ng/ul | Average ng/ul |
|---|---|---|---|---|---|
| Plug_1 | 47 | 772 | 68.2 | 68.4 | 68.3 |
| Plug_2 | - | - | 40.8 | 41 | 40.9 |
| Plug_3 | - | - | 26.4 | 26.4 | 26.4 |
NanoDrop
- Followed NanoDrop RNA Protocol
| Sample | 260/230 | 260/280 |
|---|---|---|
| Plug_1 | 1.35 | 1.98 |
| Plug_2 | 0.91 | 1.96 |
| Plug_3 | 1.05 | 1.87 |
Gel
- 1% gel run for 1 hour at 100V
- Gel Protocol
TapeStation
- Followed RNA TapeStation protocol
| Sample | RIN score |
|---|---|
| Plug_1 | 6.9 |
| Plug_2 | 7.1 |
| Plug_3 | 6.1 |