Montipora Larvae DNA/RNA Extraction 3
Using Larvae Extraction Protocol on 8 of Ariana’s Montipora Coral Larvae Stored in DNA/RNA Shield
Goal: Use optimized extraction method on 8 samples for Ariana’s project and elute the sample above D29 in only 70ul of ultrapure water instead of 100ul
Results: Good DNA and RNA quantity and quality, but mostly not good RNA purity
Take aways: The RNA purity is not very good, potentially the “dry” spin to get out any left over wash buffer is not sufficient.
| Sample | Timepoint | TP | Volume Shield in Storage | # Larvae |
|---|---|---|---|---|
| D1 | 1hpf | TP1 | 1mL | 250ul eggs |
| D5 | 5hpf | TP2 | 1mL | 100ul |
| D9 | 38hpf | TP3 | 1mL note the spreadsheet says 500ul | 50ul |
| D11 | 38hpf | TP3 | 1mL note the spreadsheet says 500ul | 50ul |
| D19 | 93hpf | TP5 | 500ul | 50ul |
| D22 | 163hpf | TP6 | 500ul | 50ul |
| D23 | 1163hpf | TP6 | 500ul | 50ul |
| D30 | 183hpf | TP9 | 300ul | 15-20 individuals |
Based off of the sample info and how many larvae were present in each tube, save fractions were made for samples D1, D5, D9, D11, D19, D22, D23 but not D30.
| Sample | Volume Shield Added at Extraction | Save Fraction? | Saved Volume |
|---|---|---|---|
| D1 | 0 | yes | 500ul |
| D5 | 0 | yes | 500ul |
| D9 | 0 | yes | 500ul |
| D11 | 0 | yes | 500ul |
| D19 | 500ul | yes | 500ul |
| D22 | 500ul | yes | 500ul |
| D23 | 500ul | yes | 500ul |
| D30 | 200ul | no | - |
Other note: I eluted the RNA of D30 in 70ul of ultrapure water instead of 100ul to try to concentrate it, we were thinking that maybe the 260/230 ratio can be worse with low concentrations of RNA. Considering how bad some of the 260/230 ratios in this extraction are regardless of concentration, I don’t know if this is a good idea. However when diluting to send to sequence, having more to dilute by might drown out some of the contamination…
Extraction
- Extraction protocol followed exactly
- Extraction proceeded with 500ul of each sample
- 500ul of DNA/RNA lysis buffer was used for each sample
- 1mL of 100% ethanol was used for each sample
- Sample D30 was eluted with 40ul ultrapure water in the first elution and 30ul in the second
QC
Qubit
- Broad Range dsDNA and Borad Range RNA Qubit protocol
- All samples were read twice
BR DNA:
| Sample | Standard 1 | Standard 2 | DNA 1 ng/ul | DNA 2 ng/ul | Average ng/ul |
|---|---|---|---|---|---|
| D1 | 174 | 19150 | too low | - | - |
| D5 | - | - | too low | - | - |
| D9 | - | - | 2.32 | 2.24 | 2.28 |
| D11 | - | - | 2.2 | 2.2 | 2.2 |
| D19 | - | - | 18.7 | 18.5 | 18.6 |
| D22 | - | - | 86.4 | 86 | 86.2 |
| D23 | - | - | 18.9 | 18.8 | 18.85 |
| D30 | - | - | 9.56 | 9.42 | 9.49 |
BR RNA:
| Sample | Standard 1 | Standard 2 | RNA 1 ng/ul | RNA 2 ng/ul | Average ng/ul |
|---|---|---|---|---|---|
| D1 | 379 | 8094 | 181 | 179 | 180 |
| D5 | - | - | 160 | 157 | 158.5 |
| D9 | - | - | 105 | 105 | 105 |
| D11 | - | - | 149 | 147 | 148 |
| D19 | - | - | 50.4 | 48.8 | 49.6 |
| D22 | - | - | 95 | 94.2 | 94.6 |
| D23 | - | - | 29.2 | 29 | 29.1 |
| D30 | - | - | 23.4 | 22.2 | 22.8 |
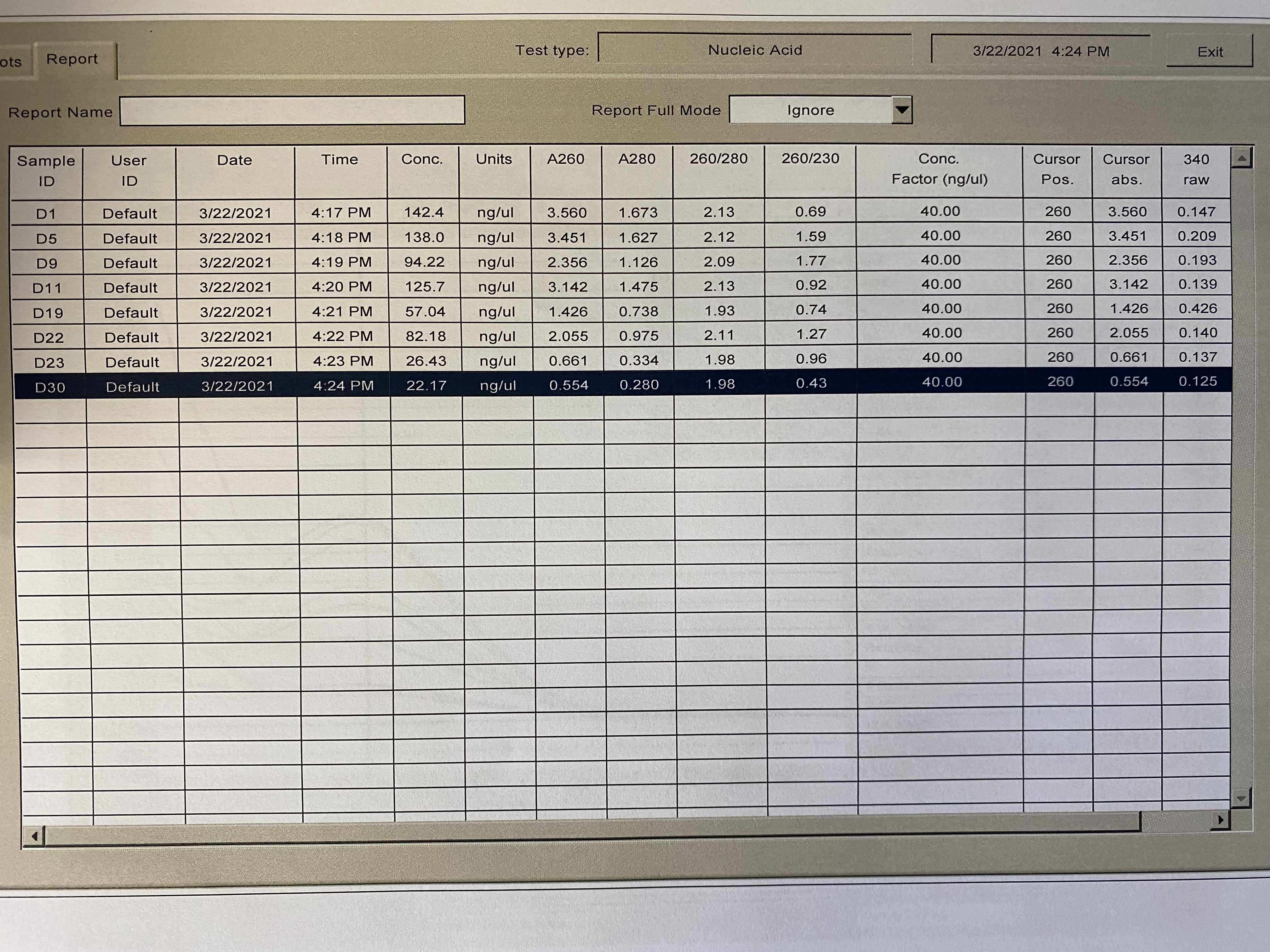
NanoDrop
- Followed NanoDrop RNA Protocol
| Sample | 260/230 | 260/280 |
|---|---|---|
| D1 | 0.69 | 2.13 |
| D5 | 1.59 | 2.12 |
| D9 | 1.77 | 2.09 |
| D11 | 0.92 | 2.13 |
| D19 | 0.74 | 1.93 |
| D22 | 1.27 | 2.11 |
| D23 | 0.96 | 1.98 |
| D30 | 0.43 | 1.98 |
Full Results:
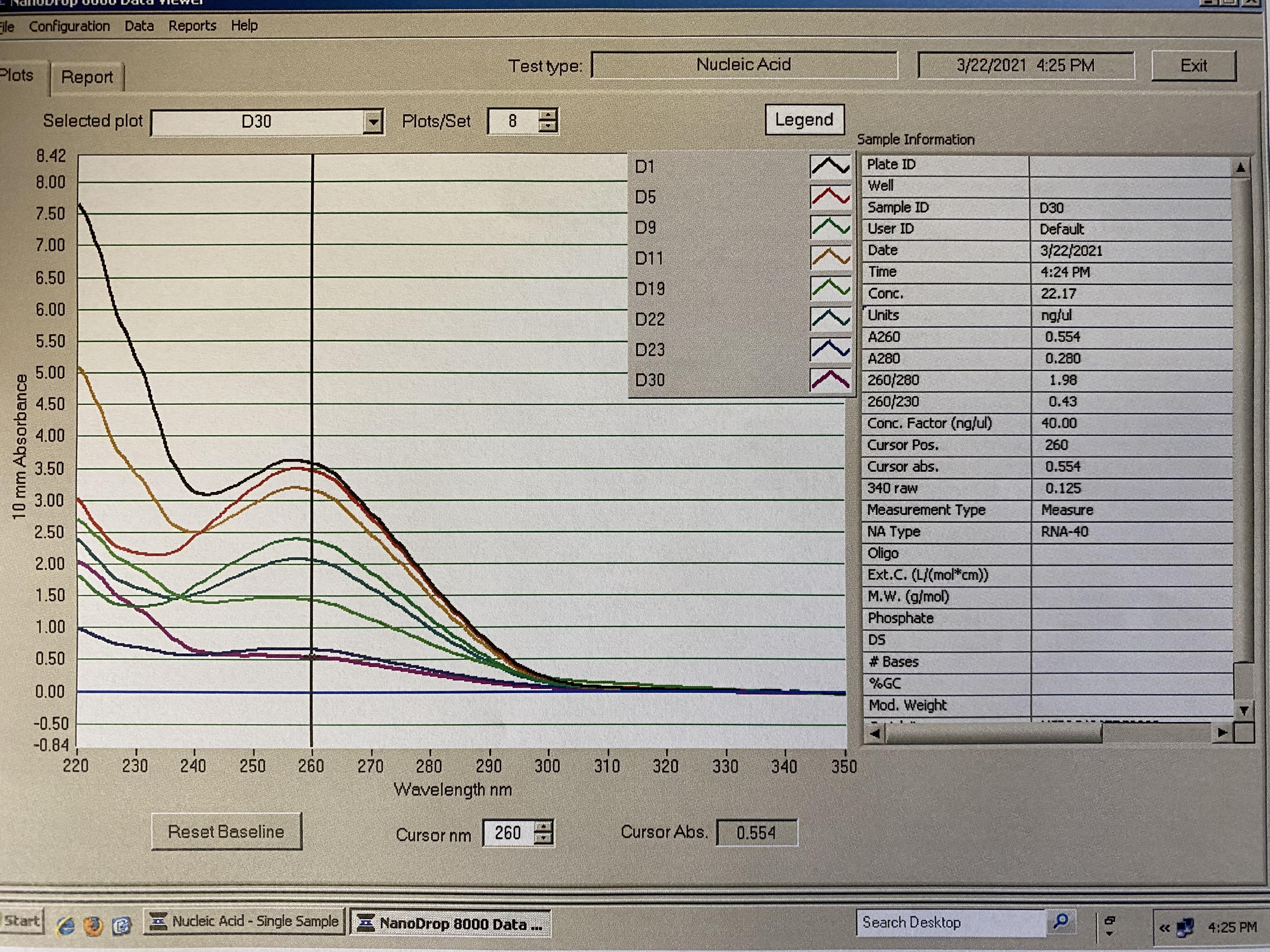
Traces:
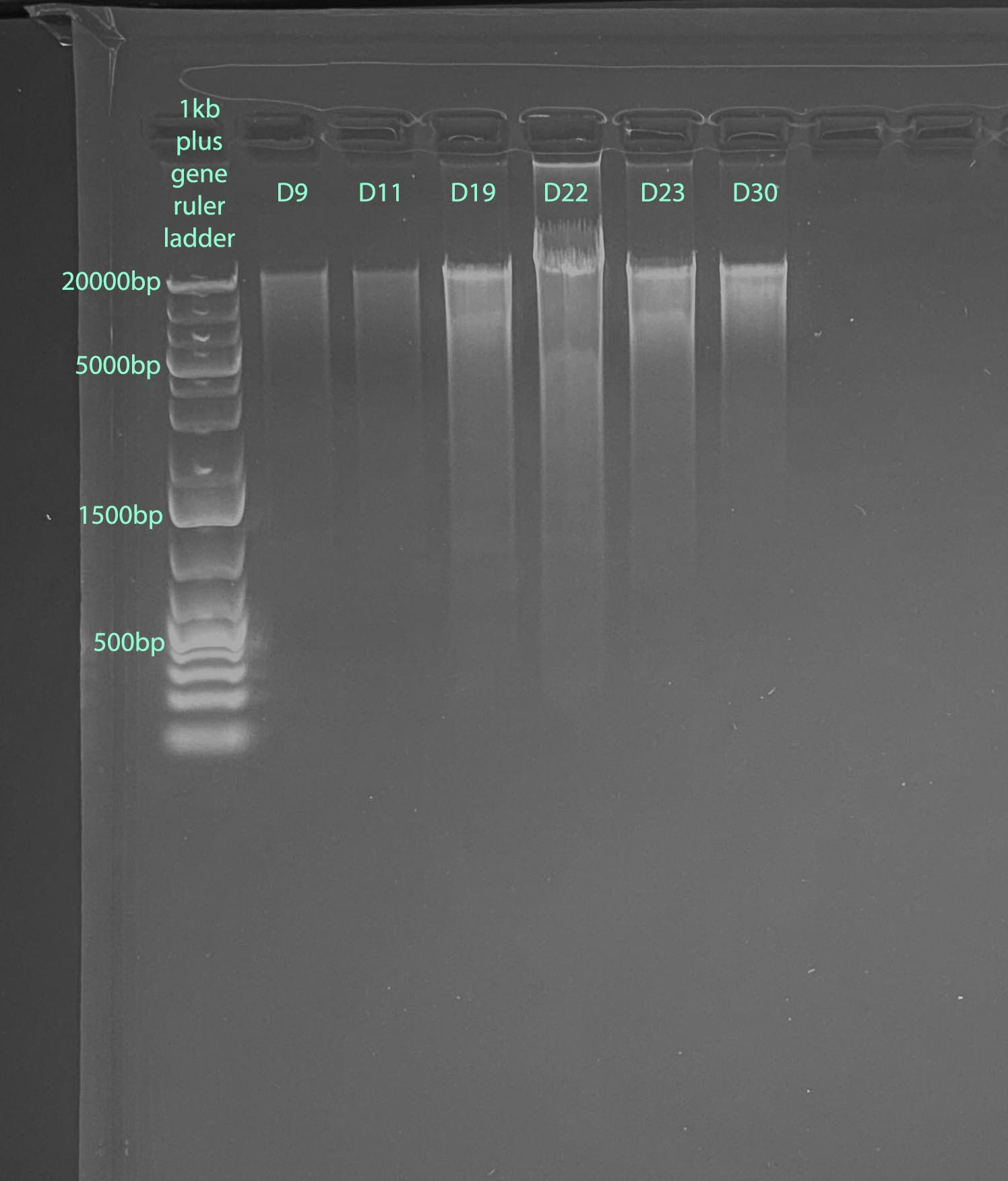
Gel
- 1% gel run for 1 hour at 100V
- Gel Protocol
TapeStation
- Followed RNA TapeStation protocol
- Two tapes were run to finish off an open tape
| Sample | RIN score |
|---|---|
| D1 | 8.1 |
| D5 | 8.3 |
| D9 | 8.7 |
| D11 | 8.7 |
| D19 | 8.7 |
| D22 | 9.0 |
| D23 | 8.4 |
| D30 | 9.1 |