Testing MiFish U and E, and COI on New Samples from IP and GSO
| Sample | DNA Concentration (µg/ul) |
|---|---|
| L | 8.02 / 8.02 |
| G2 | 3.12 / 3.2 |
| C | 0.388 / 0.39 |
| M | 7.18 / 7.16 |
| E1 | 14.7 / 14.6 |
| F | 0.89 / 0.89 |
| E2 | 9 / 9 |
| B2 | 12.7 / 12.7 |
| K1 | 1.85 / 1.85 |
| A1 | 12.8 / 12.7 |
| D2 | 14.8 / 14.9 |
| K2 | 5.82 / 5.8 |
| D1 | 4.58 / 4.58 |
| A3 | 10.4 / 10.3 |
| D1 | 4.0 / 4.0 |
| I2 | 12.7 /12.7 |
| G1 | 10.3 / 10.2 |
| I1 | 5.68 / 5.66 |
| Sample | DNA Concentration (µg/µl) |
|---|---|
| IPT-1 (30) | 36.8 /37 |
| IPT-2 (30) | 43 / 43 |
| IPT-3 (30) | 47 / 47.2 |
| IPT-4 (30) | 47 / 46.8 |
| IPT-5 (30) | 45.4 / 44.8 |
| IPT-6 (30) | 19.1 / 19.1 |
| GSO-1 (30) | 38 / 38.2 |
| GSO-2 (30) | 20.6 / 20.6 |
| GSO-3 (30) | 41 / 41 |
| GSO-4 (30) | 35 / 34.8 |
| GSO-5 (30) | 29.2 / 29.2 |
| GSO-6 (30) | 30.2 / 30.2 |
| GSO-7 (30) | 31.8 / 31.8 |
1
Testing MiFish-U at 65 C degree program. Pag # 1.
For the master mix:
- 0.7 µl of each primer (F and R) = 11.9 µl each
- 6 µl of Hot Stated Ready Mix (Kapa) = 102 µl
- 4.1 µl nuclease free water = 69.7 µl
- 1 or 2 µl of DNA template; depending of the DNA concentration of the Sample: see the table; until reach aprox. 15 ng/μl
- 12.5 µl total reaction
- 11.5 µl of master mix was pipetted in the strip tube
I tested 14 samples and 2 negatives
Gel # 1 a 1% gel was run with GelGreen for 1 hour at 110V

Only the GSO-1 worked for the GSO samples. Its probable that I use DNA template in excess (1 µl) for the IPT samples.
Gel # 2
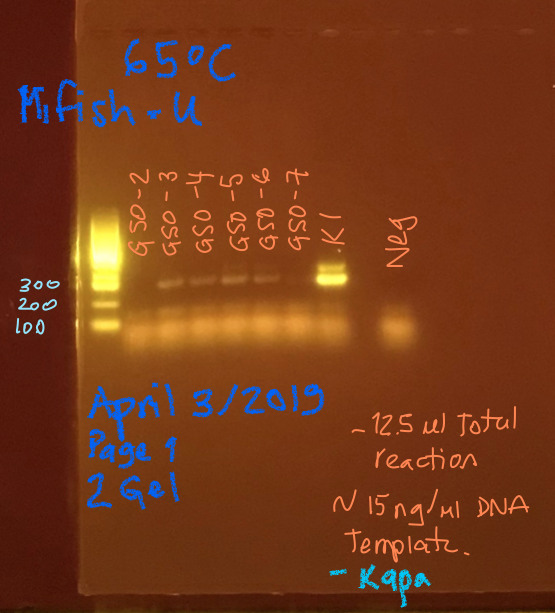
Only the GSO-2 doesn’t worked for the GSO samples and K1 works very well but with the presence os two bands.
- No signs of contamination in the negatives.
2
Testing COI; using COI program. Pag # 3.
For the master mix:
- 1 µl of each primer (F and R) = 17 µl each
- 12 µl of qiagen Mix = 212.5 µl
- 9 µl nuclease free water = 153 µl
- 1.5 µl of DNA template
- 25 µl total reaction
- 23.5 µl of master mix was pipetted in the strip tube
I tested 14 samples and 2 negatives
Gel # 1 a 1% gel was run with GelGreen for 50 min at 110V
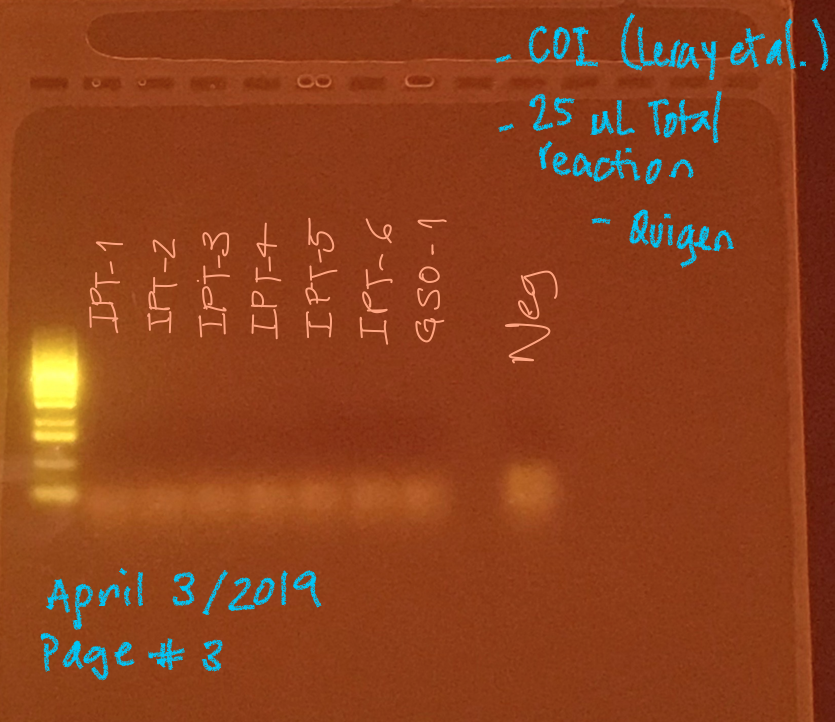
None of the samples from IPT and GSO amplified. I put DNA template in excess (1.5 µl)
Gel # 2 a 1% gel was run with GelGreen for 50 min at 110V
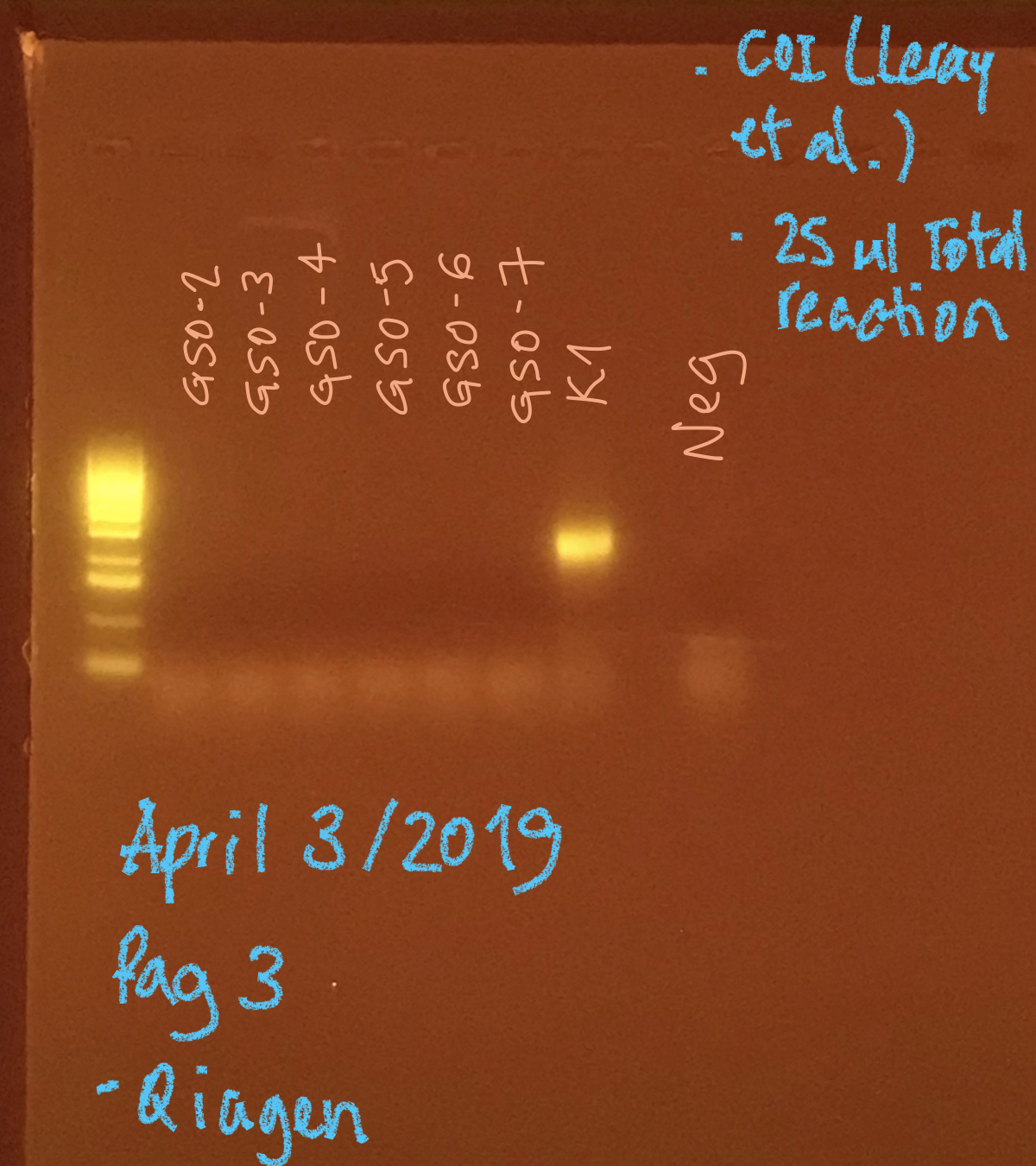
None of the samples from GSO amplified, but K1 sample worked well.
- No signs of contamination in the negatives.
3
Testing MiFish-U at 65 C degree program. Pag # 4.
For the master mix:
- 0.7 µl of each primer (F and R) = 11.9 µl each
- 6 µl of Hot Stated Ready Mix (Kapa) = 102 µl
- 4.6 µl nuclease free water = 78.2 µl
- 0.5; 1; 1.2; 1.5; 2 µl of DNA template; depending of the DNA concentration of the Sample: see the table; until reach aprox. 15 ng/μl
- 12.5 µl total reaction
- 10-11.5 µl of master mix was pipetted in the strip tube
I tested 14 samples and 2 negatives
Gel # 1 a 1% gel was run with GelGreen for 50 min at 110V
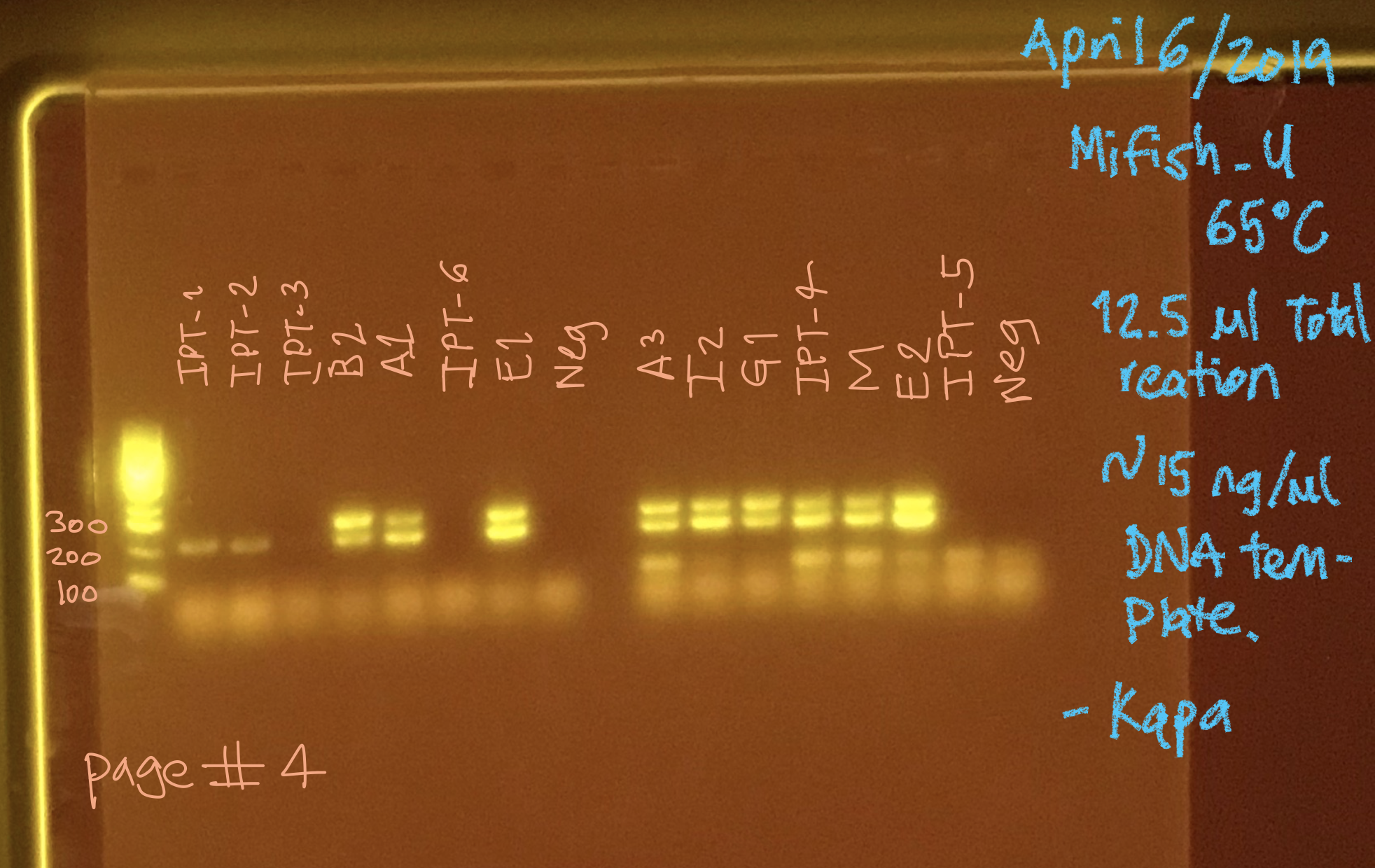
IPT-3; IPT-6; IPT-5 samples doesn’t worked. The rest of the samples (11) works well, but with the presence of a double bands.
