Test eDNA PCRs
Testing eDNA Extraction PCR Amplification with MiFISH Primers
Test 1 02-20-19
Testing MiFISH Universal and Elasmobranch primers on extracted DNA using “clean” and Test 3 and Test 4 samples using the MIFISH PCR program under CP in our thermocycler (Note that this is not the program specified in the paper)
Try volumes recommended by paper
| Sample | Tube # | Primer |
|---|---|---|
| clean | 1 | U |
| no DNA | 2 | U |
| test 3 | 3 | U |
| test 4 | 4 | U |
| clean | 5 | E |
| no DNA | 6 | E |
| test 3 | 7 | E |
| test 4 | 8 | E |
To each tube:
- 8.3µl H2O
- 1.5µl Forward primer (U or E)
- 1.5µl Reverse primer (U or E)
- 2.7µl HiFi Hot Start Ready Mix (KAPA)
- 1µl DNA sample, except for no DNA samples. Those get 1µl more H2O
PCR cycle about an hour
Ran on a 1.5% gel with 100bp ladder
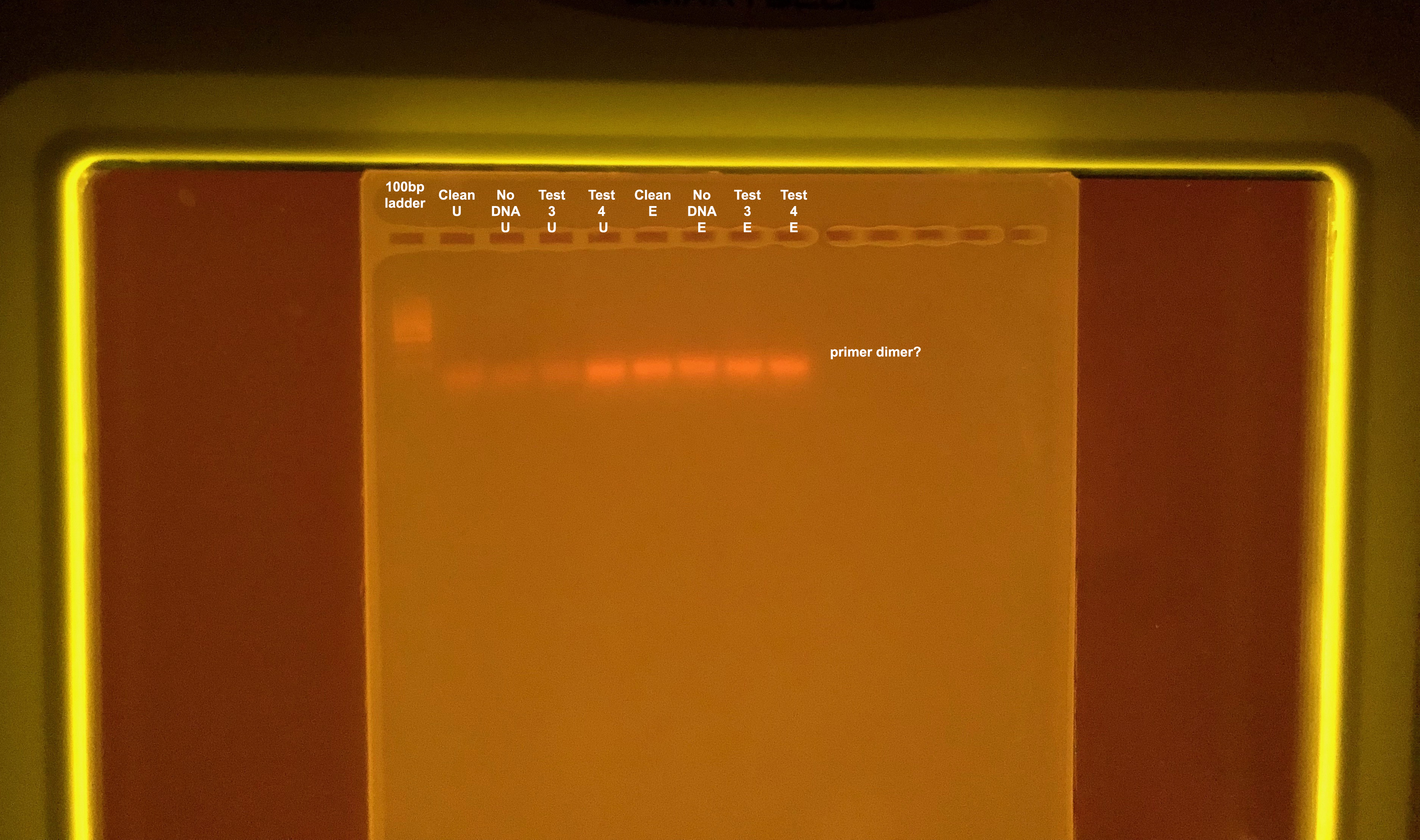
Does not work, also see that we get primer dimer.
Test 2 02-21-19
Trying less primer (1µl) and using the thermocycler program used in the paper. Programed under user MES, program MIFISH UE. Added extra 15 seconds before cycling begins at 98 degrees C for hot start
| Sample | Tube # | Primer |
|---|---|---|
| clean | 9 | U |
| control | 10 | U |
| test 3 | 11 | U |
| test 4 | 12 | U |
| clean | 13 | E |
| control | 14 | E |
| test 3 | 15 | E |
| test 4 | 16 | E |
To each tube:
- 9µl H2O
- 1µl Forward primer (U or E) not in control tubes
- 1µl Reverse primer (U or E) not in control tubes
- 3µl HiFi Hot Start Ready Mix - 1µl DNA sample, except for controls, which get 3µl more H2O
PCR cycle about an hour
Ran on a 1.5% gel with 100bp ladder
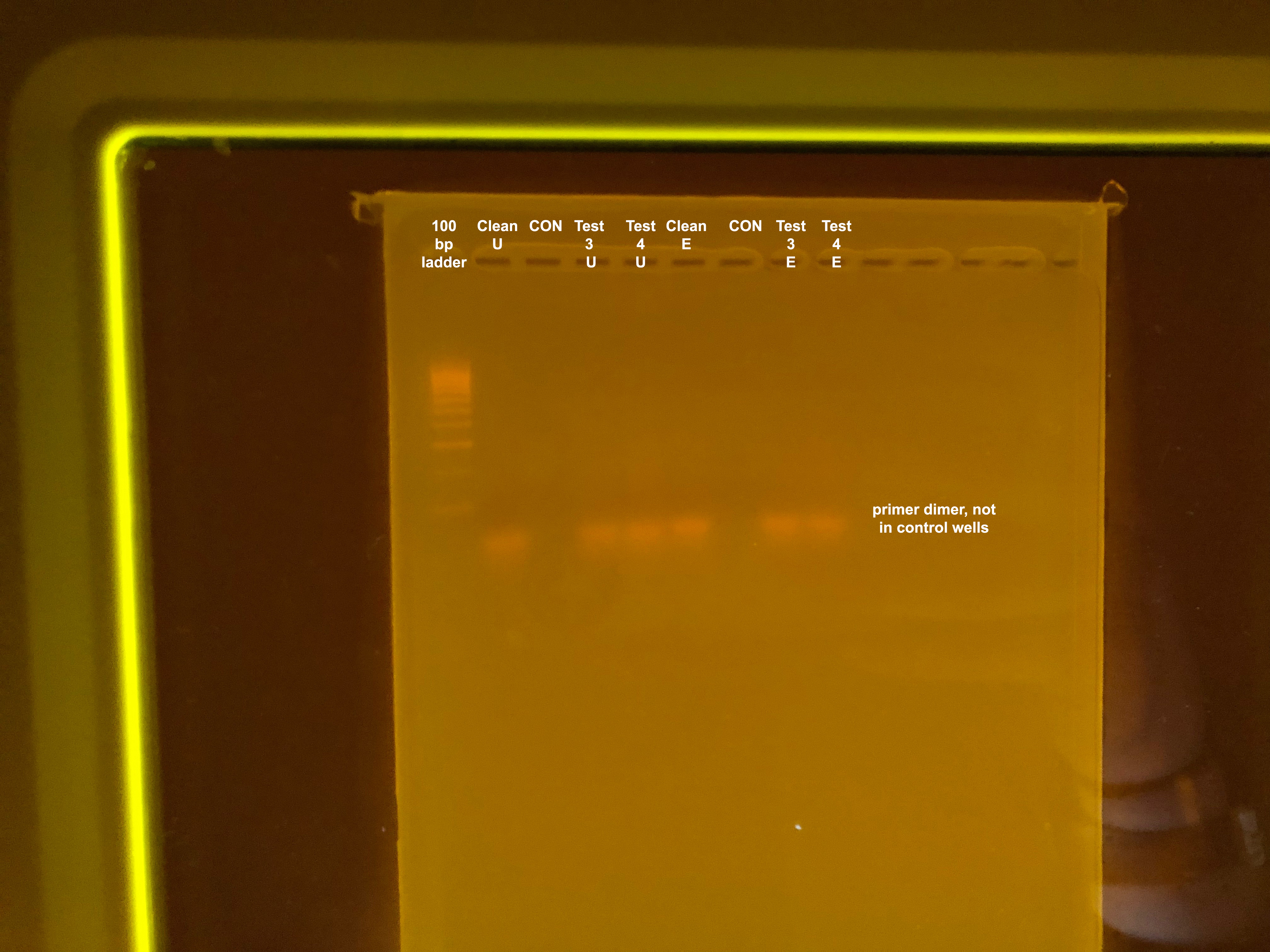
Does not work, also still getting primer dimer. Also, could not have enough DNA in the 1µl added
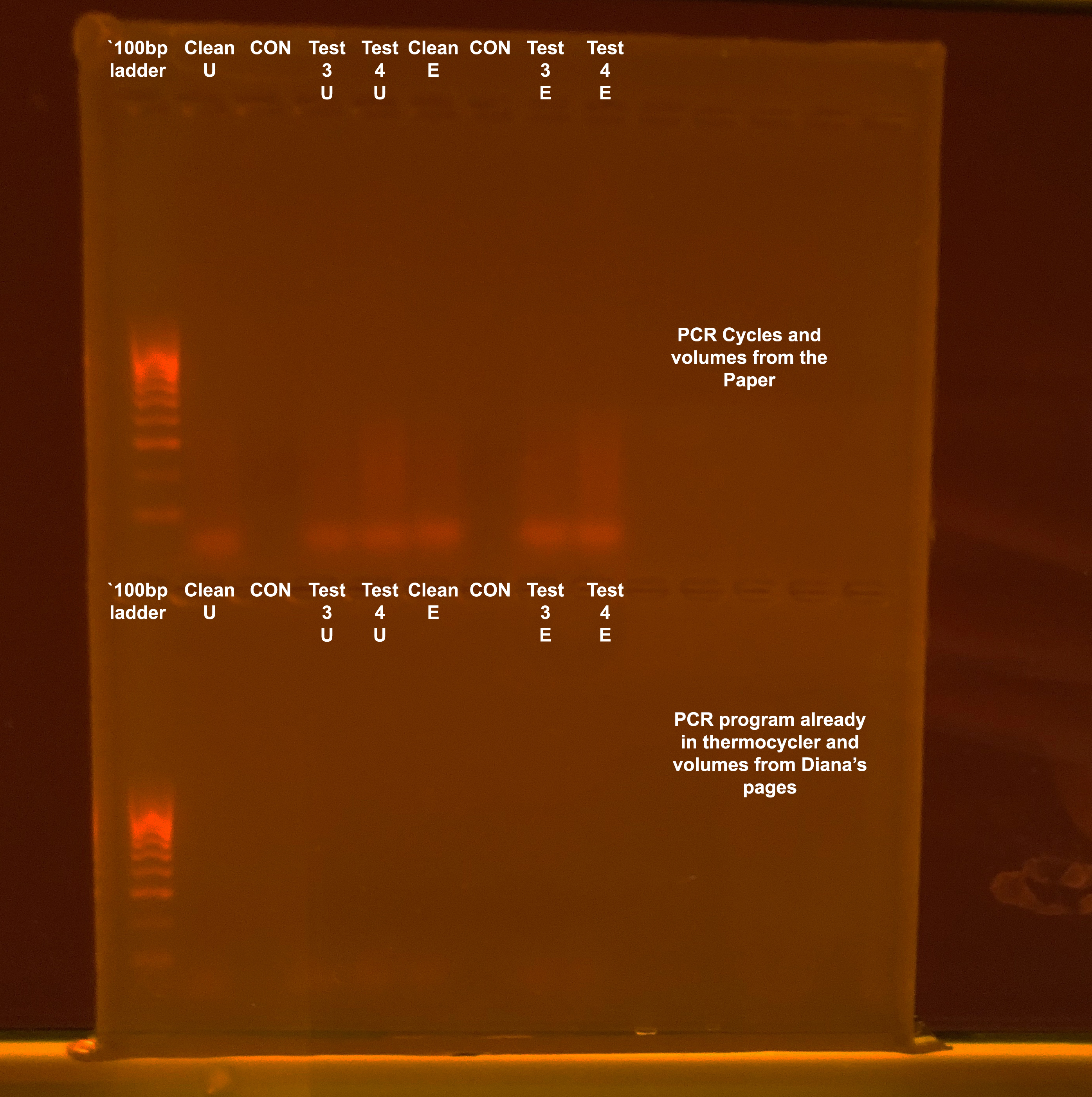
Test 3 02-25-19
Try two PCRs this time. Make new primer stocks, 10µM, and vacufuge samples to increase their concentration. Trying PCR volumes and program from the paper and PCR volumes and program from Diana already in the PCR machine and using MyTaq mix
Paper PCR
| Sample | Tube # | Primer |
|---|---|---|
| clean | 17 | U |
| control | 18 | U |
| test 3 | 19 | U |
| test 4 | 20 | U |
| clean | 21 | E |
| control | 22 | E |
| test 3 | 23 | E |
| test 4 | 24 | E |
Volumes are the same as the previous PCR volumes
Diana’s Protocol PCR
| Sample | Tube # | Primer |
|---|---|---|
| clean | 25 | U |
| control | 26 | U |
| test 3 | 27 | U |
| test 4 | 28 | U |
| clean | 29 | E |
| control | 30 | E |
| test 3 | 31 | E |
| test 4 | 32 | E |
To each tube:
- 10.9µl H2O
- 0.7µl Forward primer (U or E) not in control tubes
- 0.7µl Reverse primer (U or E) not in control tubes
- 12.5µl 2x MyTaq Mix
- 0.4µl DNA sample, except for controls, which get 2µl more H2O