Testing DiNV DNA Retention in Electroporated Cells
Electroporating and not E. coli cells with DiNV DNA and then testing for DiNV DNA presence. Also testing wether there will be more DNA inside the cells if I dilute it 1:10
Electroporation
- Prepared 4 1.5mL tubes with 975ul SOC buffer
- Using sample 22-exo
- Dilute 1:10 in 10mM tris HCl for diluted sample
- 2ul DNA and 18ul tris
- Keep on ice
- Thawed pSPIN-BAC electrocompetent cells on ice
- Brought up to Chandler lab:
- ice with bacteria and DNA
- pipettes and tips
- bacterial tip waste
- tape
- 20 1.5mL tubes
- tubes with SOC buffer
- Placed 4 SOC buffer tubes in the 30C incubator
- Placed 2 electroporation EC2 cuvettes on ice
- pSPIN-BAC DNA no electro - tube 8A and 8B
- Added 25ul pSPIN-BAC cells to a tube on ice labeled 8A
- Added 2ul 22-exo DNA
- Immediately added 975ul of 37C SOC buffer
- Transferred 490ul to a 1.5mL tube labeled “8B”
- Placed 8B on ice
- Placed 8A in the 30C shaking incubator 1 hour
- pSPIN-BAC DNA electro - tube 9A and 9B
- Added 25ul pSPIN-BAC cells to a cuvette on ice
- Added 2ul 22-exo DNA
- Electroporated on EC-2 settings
- Immediately added 975ul of 30C SOC buffer
- Transferred 490ul to two 1.5mL tube labeled “9A and 9B”
- Placed 9B on ice
- Placed 9A in the 30C shaking incubator 1 hour
- pSPIN-BAC diluted DNA no electro - tube 10A and 10B
- Added 25ul pSPIN-BAC cells to a tube on ice labeled 10A
- Added 2ul diluted 22-exo DNA
- Immediately added 975ul of 30C SOC buffer
- Transferred 490ul to a 1.5mL tube labeled “10B”
- Placed 10B on ice
- Placed 10A in the 30C shaking incubator 1 hour
- pSPIN-BAC diluted DNA electro - tube 11A and 11B
- Added 25ul pSPIN-BAC cells to a cuvette on ice
- Added 2ul diluted 22-exo DNA
- Electroporated on EC-2 settings
- Immediately added 975ul of 30C SOC buffer
- Transferred 490ul to two 1.5mL tube labeled “11A and 11B”
- Placed 11B on ice
- Placed 11A in the 30C shaking incubator 1 hour
- B tubes brought down to 4012
Washing Cells
- Centrifuged B tubes 3 min at 6,000g
- Removed supernatant
- Resuspended pellets in 200ul LB
- Repeated above steps 4 more times for 5 washes
- Removed final supernatant and froze pellets of bacteria at -80C
- For A tubes after their 1 hour incubation, I also did 5 washes with 200ul of LB
- These tubes were not frozen and immediately went to DNA extraction
DNA extraction
- Used both the A and B tubes of bacteria cell pellets
- B tubes were taken from -80 and thawed on ice
- DNA extraction was done exactly as described in a previous post
PCRs on DNA Extracts
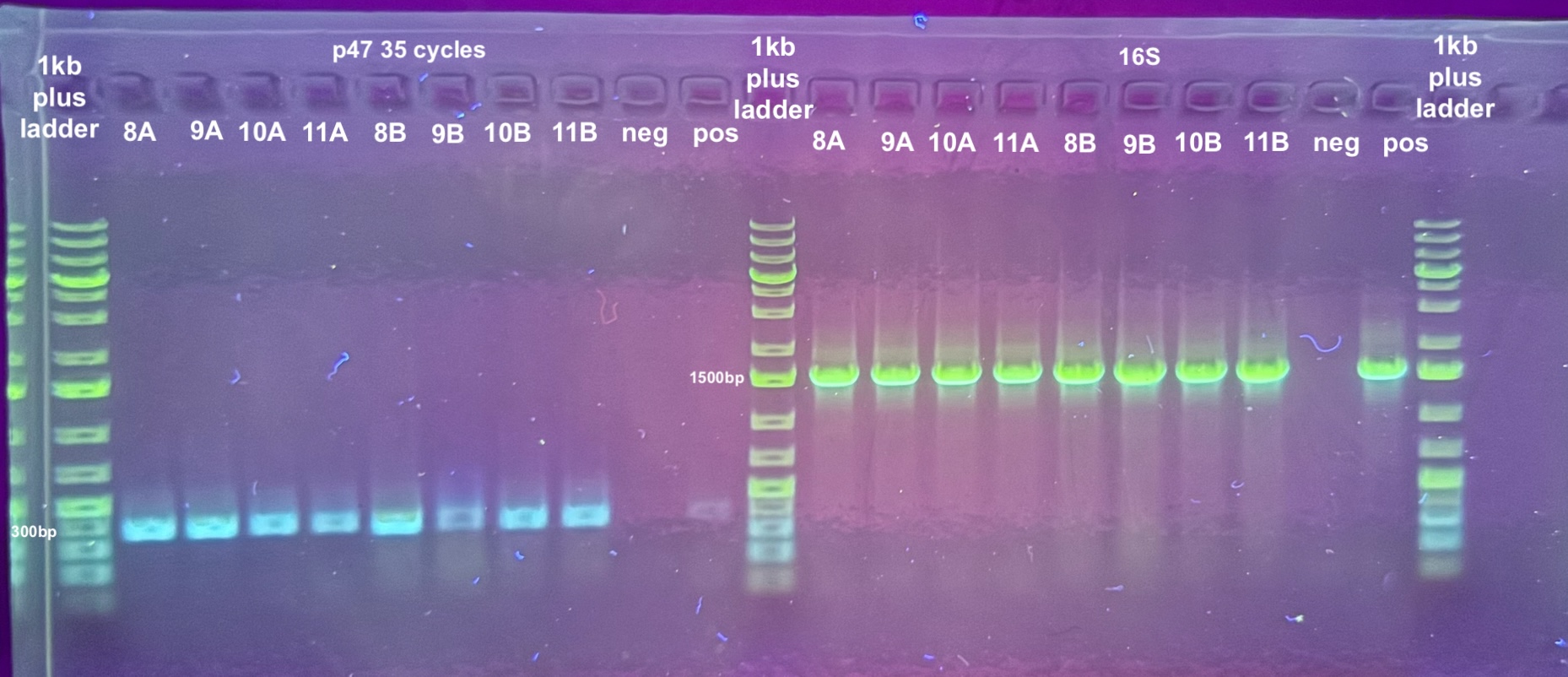
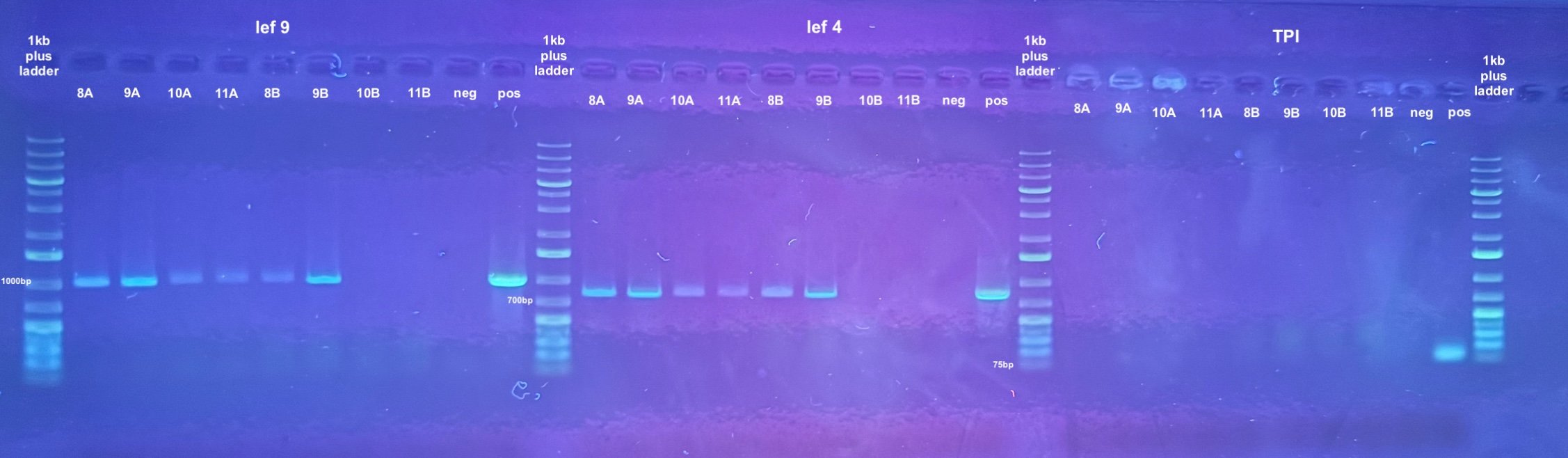
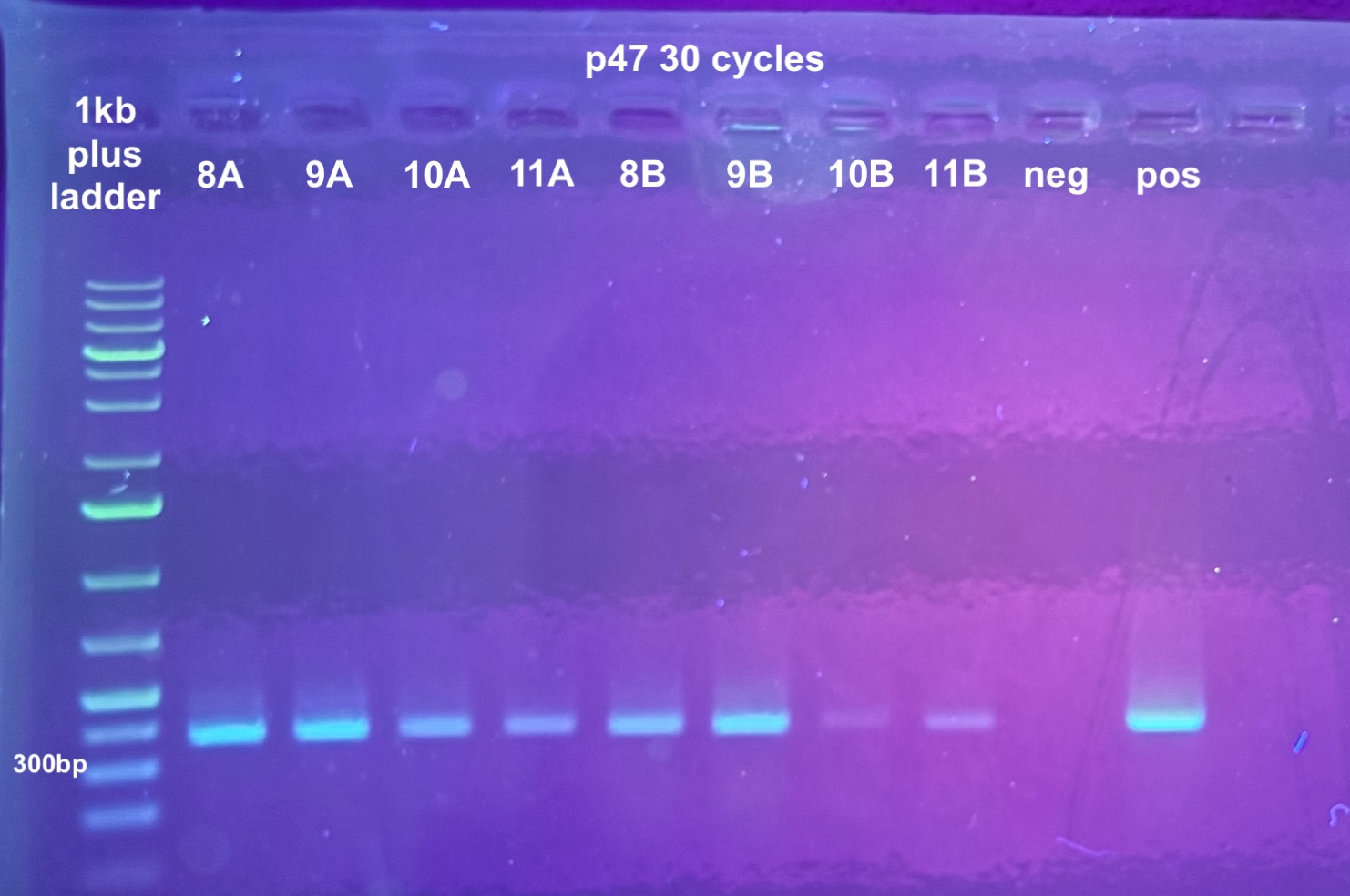
- The point was test all of the DNA samples for if they have retained DiNV DNA with PCR
- 5 PCRs were run on the samples, and the process followed the general PCR protocol completely. Master mix volumes are listed here:
| reagent | p47 | 16S | lef 9 | lef 4 | TPI |
|---|---|---|---|---|---|
| GoTaq | 53ul | 53ul | 53ul | 53ul | 53ul |
| F primer | 2.65ul | 2.65ul | 2.65ul | 2.65ul | 2.65ul |
| R primer | 2.65ul | 2.65ul | 2.65ul | 2.65ul | 2.65ul |
| molecular grade water | 37.1ul | 37.1ul | 37.1ul | 37.1ul | 37.1ul |
- All PCR programs were run for 30 cycles except for p47 which was then re-run for 30 cycles, and program information can be found here
- 1% gels were run at 90V for 45 minutes to resolve the bands: