More Colony PCR on Plates from Electroporation of pSPIN-BAC E. coli with DiNV DNA Attempt 1
I decided to pick at least 10 more colonies of each of the plates prepared after electroporation and do colony PCR on them. The first set did not show much promise of DiNV. I used this Addgene blog for help on how to plan the PCRs. Rob suggested doing a positive control PCR and using 16S (bacterial) to see if the colony PCR works at all. I did not pick any colonies from the negative control plates for this time.
For each colony, I picked it with an autoclaved pipette tip and swirled it in the tube of 5ul of water for a few seconds:
| number | plate | treatment |
|---|---|---|
| 28 | 7 | DiNV DNA and electroporation |
| 29 | 7 | DiNV DNA and electroporation |
| 30 | 7 | DiNV DNA and electroporation |
| 31 | 7 | DiNV DNA and electroporation |
| 32 | 7 | DiNV DNA and electroporation |
| 33 | 7 | DiNV DNA and electroporation |
| 34 | 7 | DiNV DNA and electroporation |
| 35 | 7 | DiNV DNA and electroporation |
| 36 | 7 | DiNV DNA and electroporation |
| 37 | 7 | DiNV DNA and electroporation |
| 38 | 8 | DiNV DNA and electroporation |
| 39 | 8 | DiNV DNA and electroporation |
| 40 | 8 | DiNV DNA and electroporation |
| 41 | 8 | DiNV DNA and electroporation |
| 42 | 8 | DiNV DNA and electroporation |
| 43 | 8 | DiNV DNA and electroporation |
| 44 | 8 | DiNV DNA and electroporation |
| 45 | 8 | DiNV DNA and electroporation |
| 46 | 8 | DiNV DNA and electroporation |
| 47 | 8 | DiNV DNA and electroporation |
| 48 | 10 | DiNV DNA and electroporation |
| 49 | 10 | DiNV DNA and electroporation |
| 50 | 10 | DiNV DNA and electroporation |
| 51 | 10 | DiNV DNA and electroporation |
| 52 | 10 | DiNV DNA and electroporation |
| 53 | 10 | DiNV DNA and electroporation |
| 54 | 10 | DiNV DNA and electroporation |
| 55 | 10 | DiNV DNA and electroporation |
| 56 | 10 | DiNV DNA and electroporation |
| 57 | 10 | DiNV DNA and electroporation |
| 58 | 11 | DiNV DNA and electroporation |
| 59 | 11 | DiNV DNA and electroporation |
| 60 | 11 | DiNV DNA and electroporation |
| 61 | 11 | DiNV DNA and electroporation |
| 62 | 11 | DiNV DNA and electroporation |
| 63 | 11 | DiNV DNA and electroporation |
| 64 | 11 | DiNV DNA and electroporation |
| 65 | 11 | DiNV DNA and electroporation |
| 66 | 11 | DiNV DNA and electroporation |
| 67 | 11 | DiNV DNA and electroporation |
| 68 | 12 | DiNV DNA and electroporation |
| 69 | 12 | DiNV DNA and electroporation |
| 70 | 12 | DiNV DNA and electroporation |
| 71 | 12 | DiNV DNA and electroporation |
| 72 | 12 | DiNV DNA and electroporation |
| 73 | 12 | DiNV DNA and electroporation |
| 74 | 12 | DiNV DNA and electroporation |
| 75 | 12 | DiNV DNA and electroporation |
| 76 | 12 | DiNV DNA and electroporation |
| 77 | 12 | DiNV DNA and electroporation |
| 78 | 12 | DiNV DNA and electroporation |
| 79 | 12 | DiNV DNA and electroporation |
| 80 | 13 | DiNV DNA and electroporation |
| 81 | 13 | DiNV DNA and electroporation |
| 82 | 13 | DiNV DNA and electroporation |
| 83 | 13 | DiNV DNA and electroporation |
| 84 | 13 | DiNV DNA and electroporation |
| 85 | 13 | DiNV DNA and electroporation |
| 86 | 13 | DiNV DNA and electroporation |
| 87 | 13 | DiNV DNA and electroporation |
| 88 | 13 | DiNV DNA and electroporation |
| 89 | 13 | DiNV DNA and electroporation |
| 90 | 13 | DiNV DNA and electroporation |
| 91 | 13 | DiNV DNA and electroporation |
| 92 | 9 | DiNV DNA and electroporation |
| 93 | 9 | DiNV DNA and electroporation |
| 94 | 9 | DiNV DNA and electroporation |
| 95 | 9 | DiNV DNA and electroporation |
| 96 | 9 | DiNV DNA and electroporation |
| 97 | 9 | DiNV DNA and electroporation |
| 98 | 9 | DiNV DNA and electroporation |
| 99 | 9 | DiNV DNA and electroporation |
| 100 | 9 | DiNV DNA and electroporation |
| 101 | 9 | DiNV DNA and electroporation |
| 102 | 9 | DiNV DNA and electroporation |
| 103 | 9 | DiNV DNA and electroporation |
| 104 | 9 | DiNV DNA and electroporation |
| 105 | 9 | DiNV DNA and electroporation |
| 106 | 9 | DiNV DNA and electroporation |
| 107 | 9 | DiNV DNA and electroporation |
- Thawed reagents and primers on ice, vortexed and spun down
- Prepared master mixes on ice:
| reagent | 16S volume | p47 volume |
|---|---|---|
| GoTaq | 450ul | 450ul |
| Forward primer | 22.5ul | 22.5ul |
| Reverse primer | 22.5ul | 22.5ul |
| molecular grade water | 315ul | 315ul |
- Vortexed and spun down master mixes
- Aliquoited 9ul of master mix to strip tubes
- Added 1ul of colony-water mix to each tube
- Added 1ul of molecular grade water, and 1ul of positive DNA for the p47 primers to their respective tubes
- Vortexed and spun down
- Placed tubes in the p47 and 16S PCR programs, the p47 program cycling 35 times, the 16S program only 30
Initially I only ran the p47 results on a gel because I expected the 16S to run with no issue. Because of gel staining issues I had to run these samples across 3 separate gels:
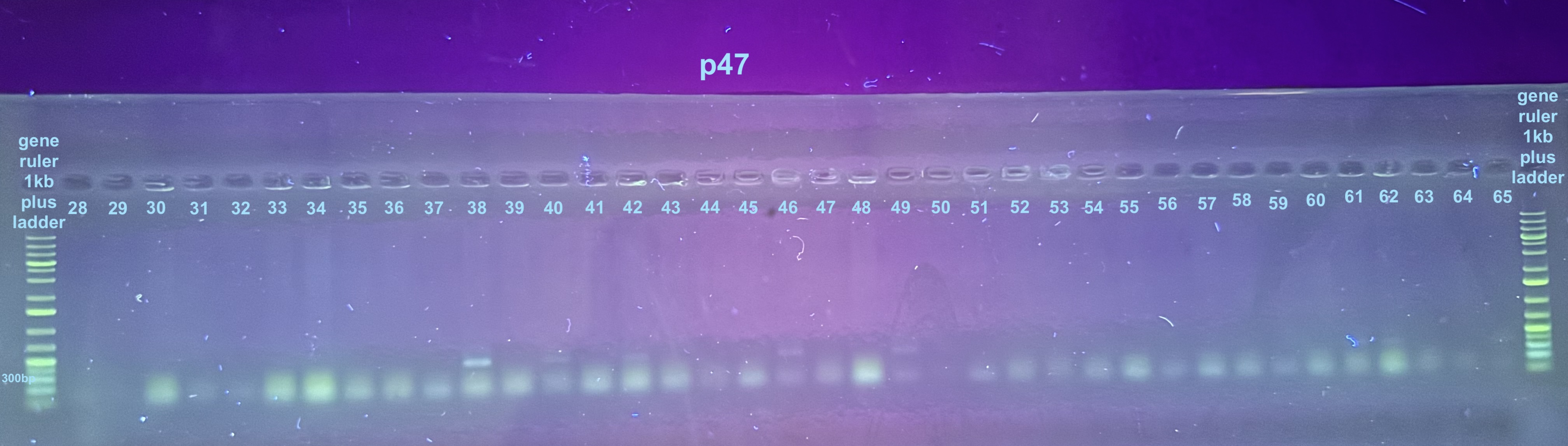
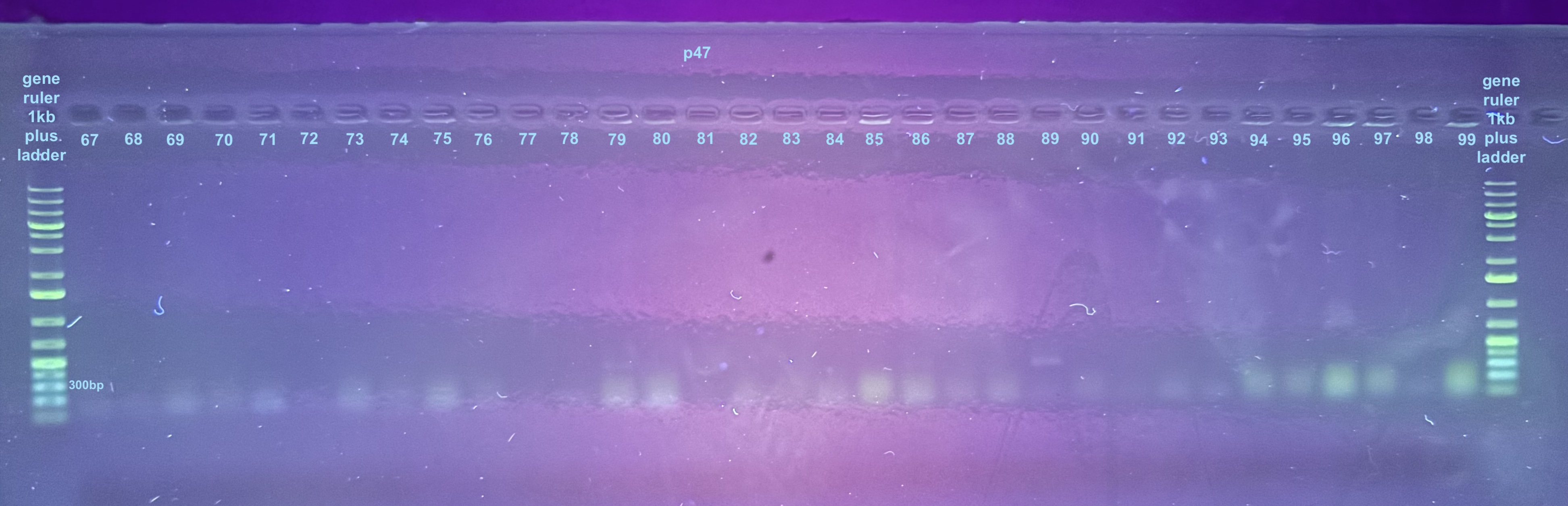
For the third gel the samples to look at are 100-107 and the controls only:
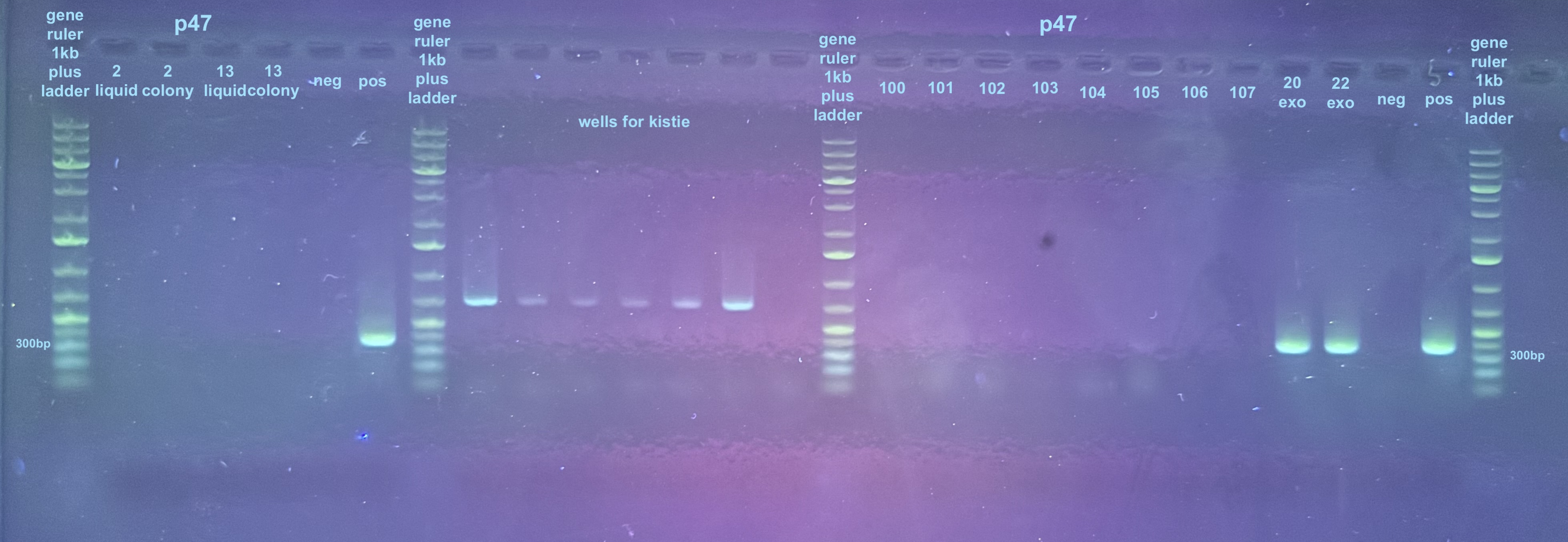
A couple samples looked potentially promising…