Taking Samples and Fluid Changing Flasks at the 15 Week Mark for Experimental DiNV Evolution in Dinn Cells (~106 days) and Extracting DNA from 1 50ul Sample Per Replicate Flask, and Checking Samples for Virus Presence
Sampling 20240110
Cells were inoculated on the 26th of Sept see post here. There are 3 replicate flasks that have been left to grow and get infected in the 23C incubator for 106 days. I am only removing supernatant for samples, the cells are not getting passed throughout this experiment, the only thing that happens is a tri-weekly fluid change (once every three weeks). In the six week sampling I took 7 50ul supernatant and 1 1mL supernatant sample per replicate flask.
All steps took place in the cell culture hood
- Using 10% FBS, 4% mushroom Schneider’s medium with antibiotics in the hood to room temperature
- Prepared 1.5mL tubes to take samples
- Removed 7 samples of 50ul and 1 sample of 1000ul from each flask before changing fluid and placed into the 1.5mL tubes:
| tube number | day sampled | date sampled | sample volume | replicate tube | flask from |
|---|---|---|---|---|---|
| 90 | day 106 | 20240110 | 50ul | 1 | A |
| 91 | day 106 | 20240110 | 50ul | 2 | A |
| 92 | day 106 | 20240110 | 50ul | 3 | A |
| 93 | day 106 | 20240110 | 50ul | 4 | A |
| 94 | day 106 | 20240110 | 50ul | 5 | A |
| 95 | day 106 | 20240110 | 50ul | 6 | A |
| 96 | day 106 | 20240110 | 50ul | 7 | A |
| 97 | day 106 | 20240110 | 1mL | NA | A |
| 98 | day 106 | 20240110 | 50ul | 1 | B |
| 99 | day 106 | 20240110 | 50ul | 2 | B |
| 100 | day 106 | 20240110 | 50ul | 3 | B |
| 101 | day 106 | 20240110 | 50ul | 4 | B |
| 102 | day 106 | 20240110 | 50ul | 5 | B |
| 103 | day 106 | 20240110 | 50ul | 6 | B |
| 104 | day 106 | 20240110 | 50ul | 7 | B |
| 105 | day 106 | 20240110 | 1mL | NA | B |
| 106 | day 106 | 20240110 | 50ul | 1 | C |
| 107 | day 106 | 20240110 | 50ul | 2 | C |
| 108 | day 106 | 20240110 | 50ul | 3 | C |
| 109 | day 106 | 20240110 | 50ul | 4 | C |
| 110 | day 106 | 20240110 | 50ul | 5 | C |
| 111 | day 106 | 20240110 | 50ul | 6 | C |
| 112 | day 106 | 20240110 | 50ul | 7 | C |
| 113 | day 106 | 20240110 | 1mL | NA | C |
- Removed 1.5mL of remaining medium in each flask and discarded it into a 15mL conical. The conical was placed in the autoclave trash when full (should contain virus)
- Added 3mL of 10% FBS, 4% mushroom Schneider’s medium with antibiotics to each flask gently
- Gently rocked flasks to distribute the medium
- Placed flasks back in the 23C incubator for another 3 weeks
- Sample tubes were frozen at -20
Sample information can be found here
DNA Extractions
For these DNA extractions I added in 3 samples from the week 12 sampling because I hadn’t done extractions on them because of the winter holidays.
Samples:
| sample # | day | flask |
| 66 | day 84 | A |
| 74 | day 84 | B |
| 82 | day 84 | C |
| 90 | day 106 | A |
| 98 | day 106 | B |
| 106 | day 106 | C |
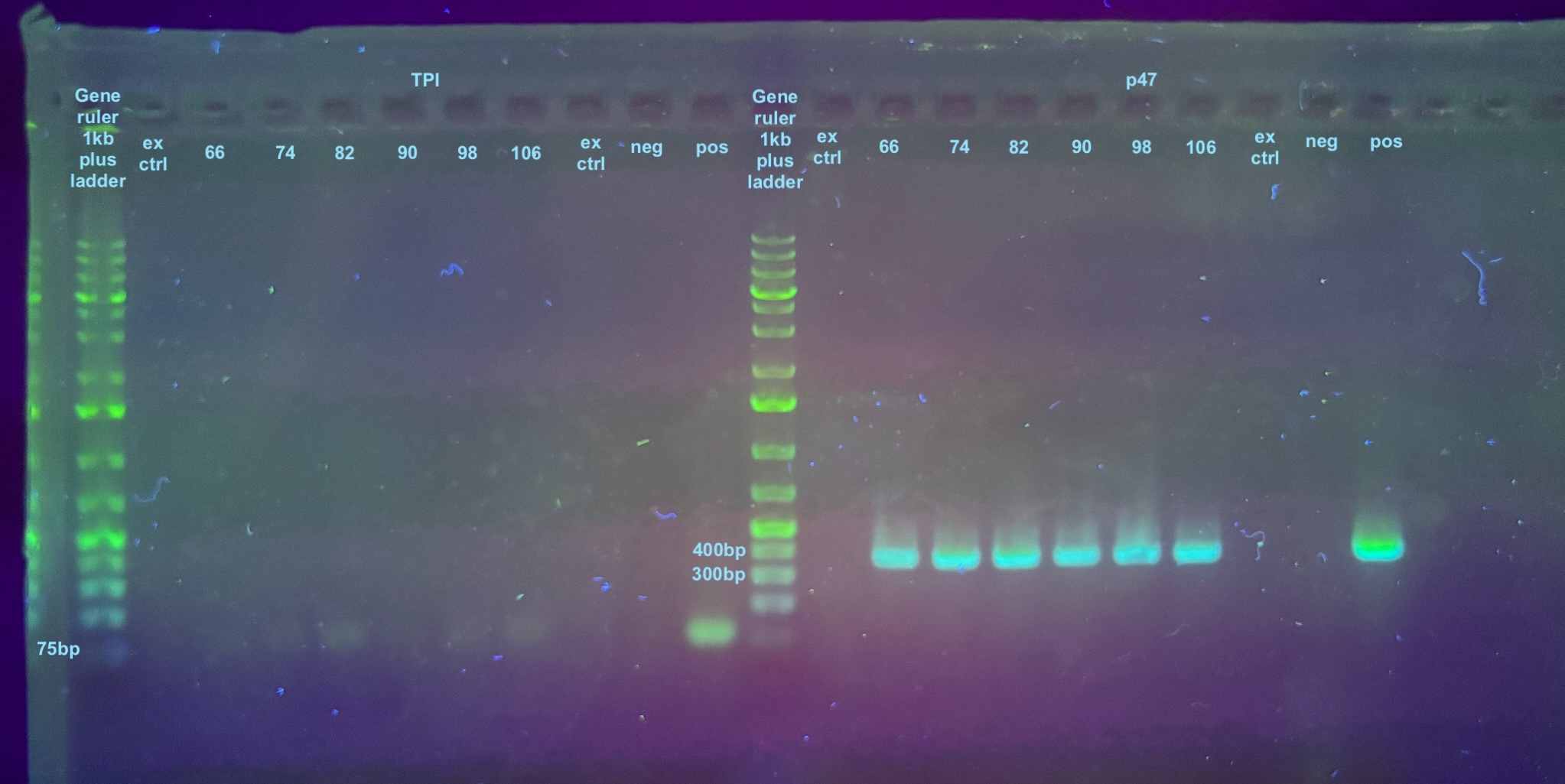
All samples were thawed on ice, this general protocol was used, and filter tips were used at all times. Since all samples were presumed positive for DiNV, there was no specific order to them. 2 Extraction controls were done at the same time as well.
PCRs
I did not dilute these samples for PCR. The PCR programs were set to 30 cycles. Information on the primers and their programs can be found here, but the TPI program had an extension time of 30 seconds.
Gel 20240111
1% gel ran for 35 minutes, 90V.