Picking 3 Colonies to Make Glycerol Stocks for the pSPIN BAC and Checking for Kanamycin and Chloramphenicol Resistance
20231215 Glycerol Stocks
The bacteria (eletroporated here) had grown on the Kanamycin resistance plates, so I felt pretty sure that the colonies had taken up the plasmid, but I chose 3 colonies, 1 from each plate to make glycerol stocks just in case some of them didn’t seem to work.
Colonies chosen here:
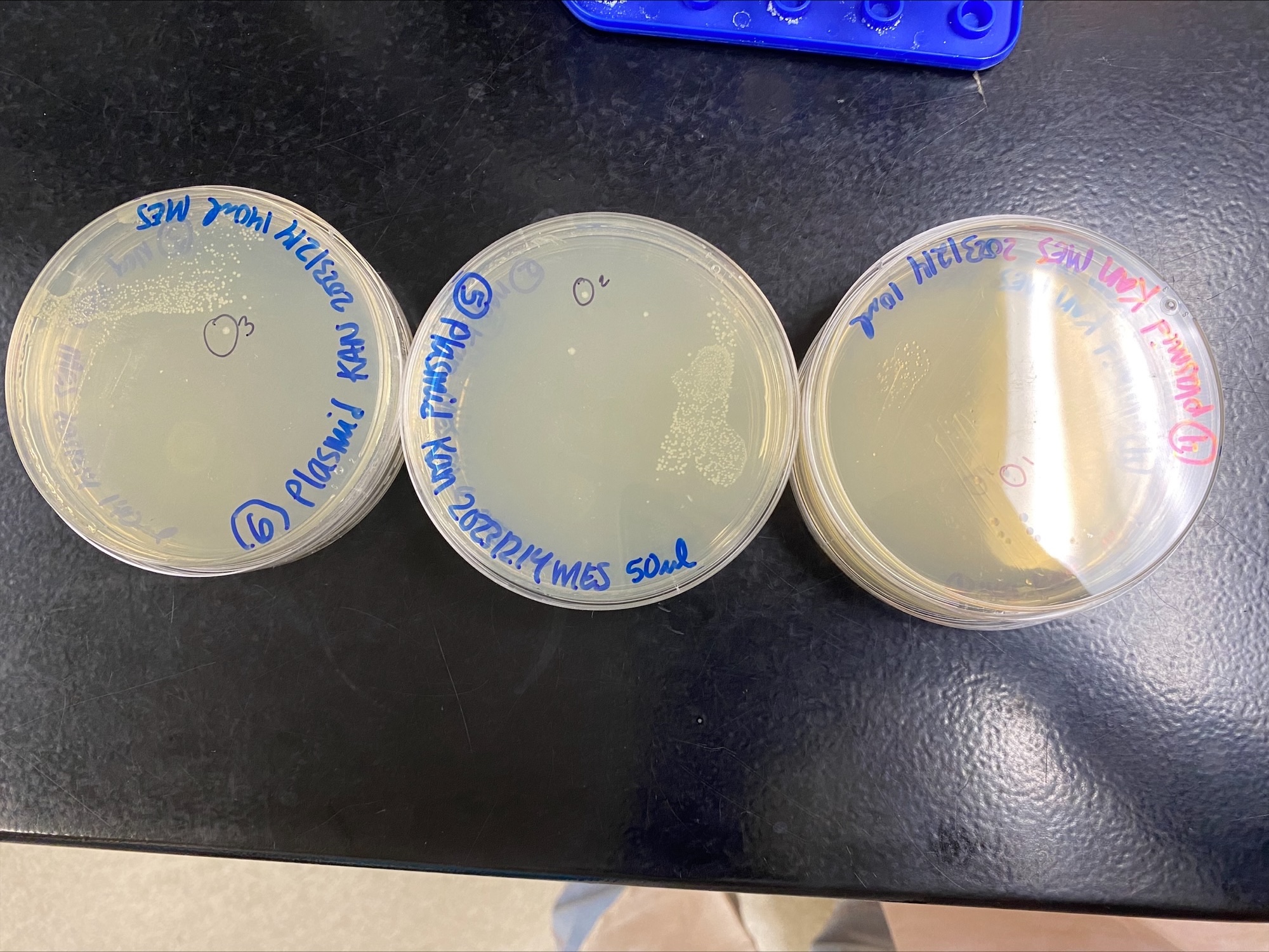
Three slightly less then 2mL LB flasks were made per colony. Because the volume was less than 2mL (the usual, next time I will prepare my own 2mL flasks), I used 0.9ul of stock Kanamycin in each flask. Usually for 2mL it is 1ul. The colonies were picked with autoclaved tips and dropped into the flasks, each colony got picked 3 times (you only need a small amount of bacteria to seed these flasks). Flasks were then grown overnight at 37C in the shaking incubator.
20231216 Finishing Stocks and Plating
From the stocks I planned to test whether the bacteria also had chloramphenicol resistance by plating each stock on Kan, Chlor, and Kan+Chlor plates.
- All flasks had grown good amounts of bacteria overnight
- 2 cryotubes were made for each colony for stocks:
- 144 - colony 1
- 145 - colony 2
- 146 - colony 3
- Each cryotube gets 750ul of culture and 750ul of 50% sterile filtered glycerol
- Stocks were pipette mixed and stored in the -80 when not using
- 9 plates were warmed in the 37C incubator
- 6 plates would get Kan and 6 plates would get Chlor, with 3 overlapping
- I know these all grow with Kan but that is a good positive control
- For the Kanamycin I wanted to spread 20ul on each plate:
- 45ul of stock Kanamycin
- 75ul of molecular grade water
- Pipette mixed, and spread on the plates with a sterile spreader
- Let these plates absorb for ~5 minutes before adding on Chloramphenicol
- For the Chloramphenicol plates I also wanted 20ul to spread on the plate and the same dilution factor, 20ug/mL on 25mL plates
- 45ul of stock Chloramphenicol
- 75ul of 100% ethanol
- Pipette mixed, and spread on the plates with a sterile spreader
- After the antibiotics were set in each plate was spread with their planned colony using a sterile loop
- The plates were left to grow upsidedown in the 37C incubator overnight
- Plates checked in the morning of the 17th, colonies 1 and 2 grew on every plate, colony 3 did not grow on plates with Chloramphenicol:
Colony 1 Chloramphenicol:
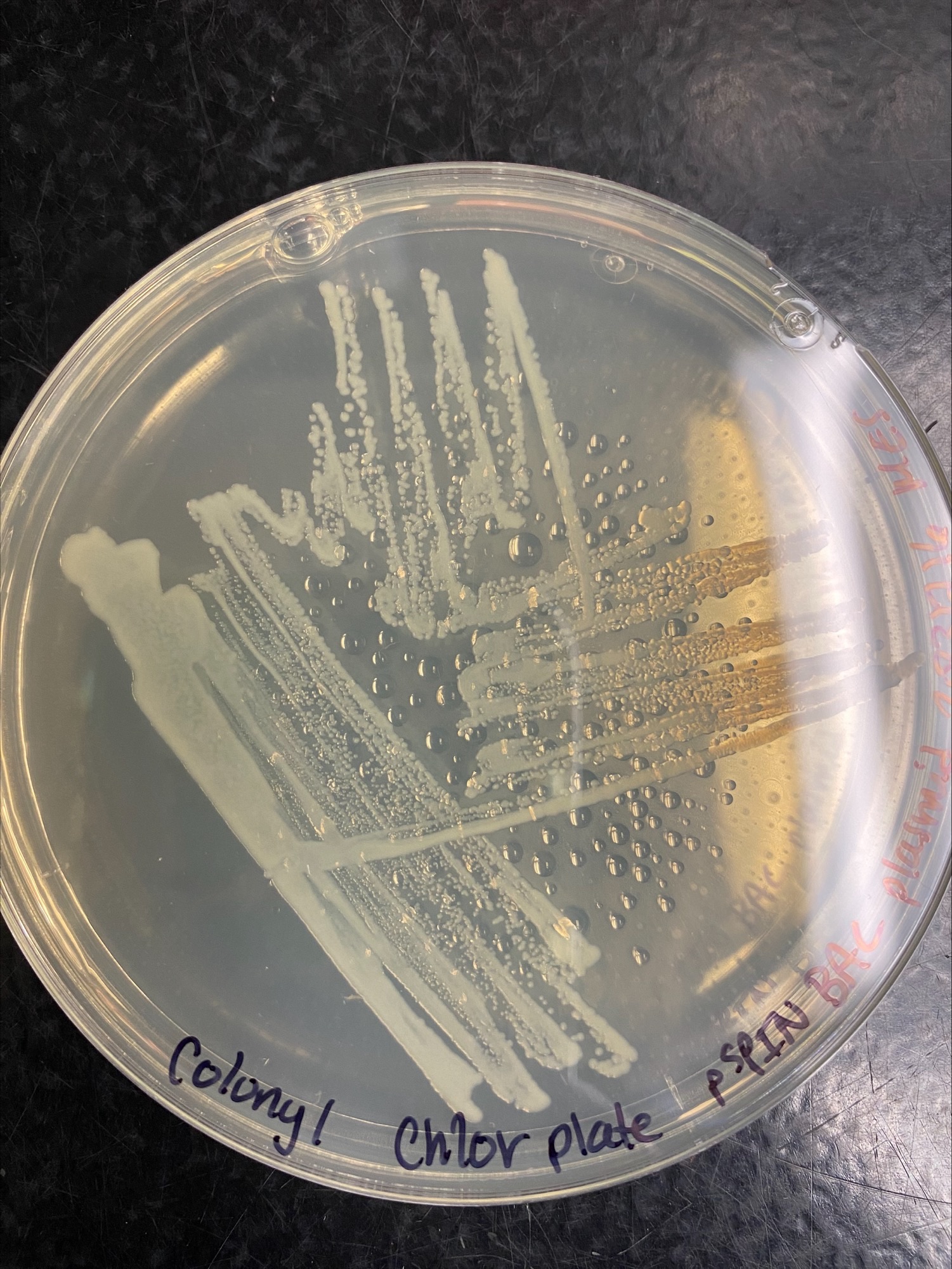 Colony 1 Kanamycin:
Colony 1 Kanamycin:
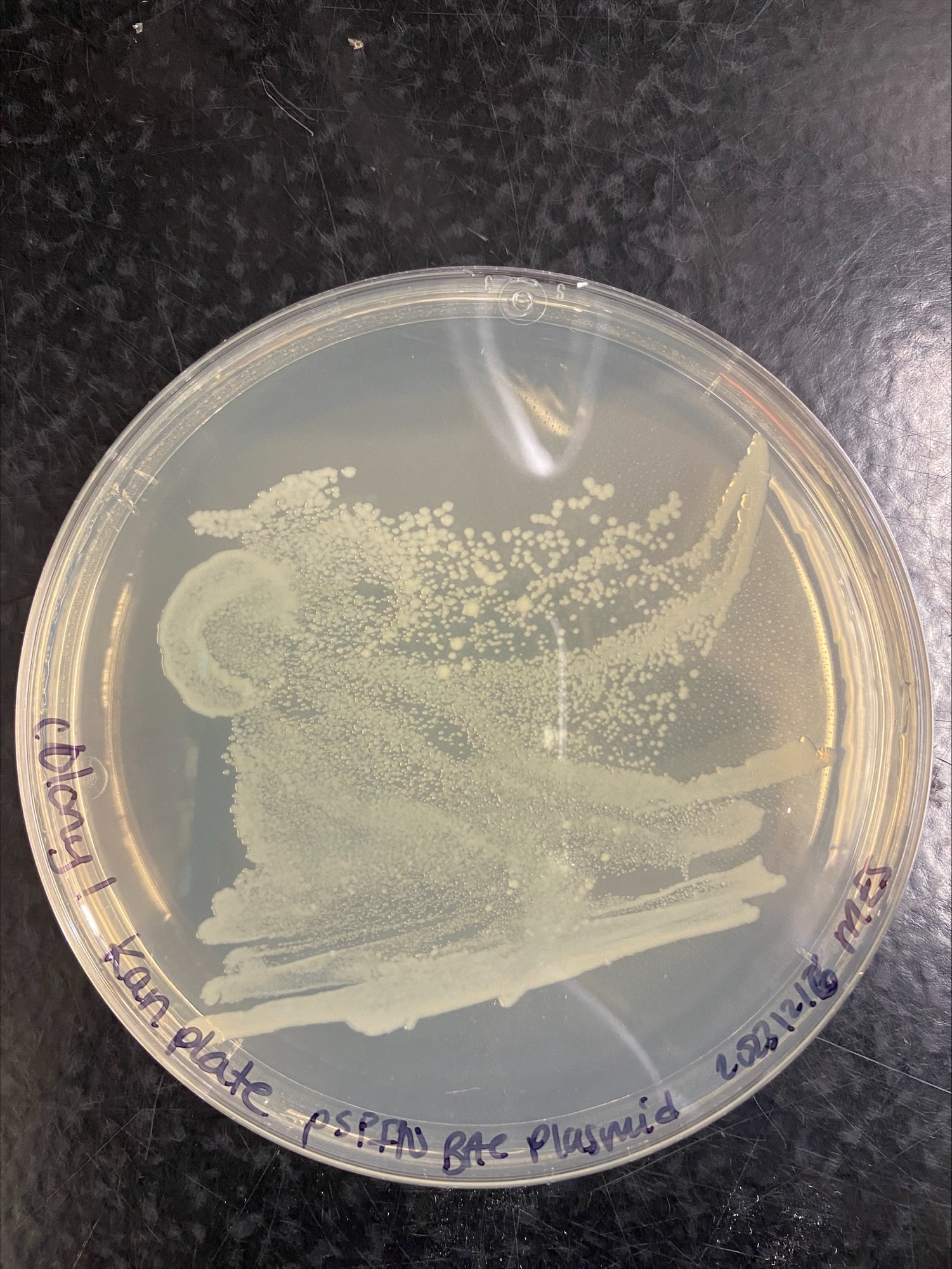 Colony 1 Chloramphenicol and Kanamycin:
Colony 1 Chloramphenicol and Kanamycin:
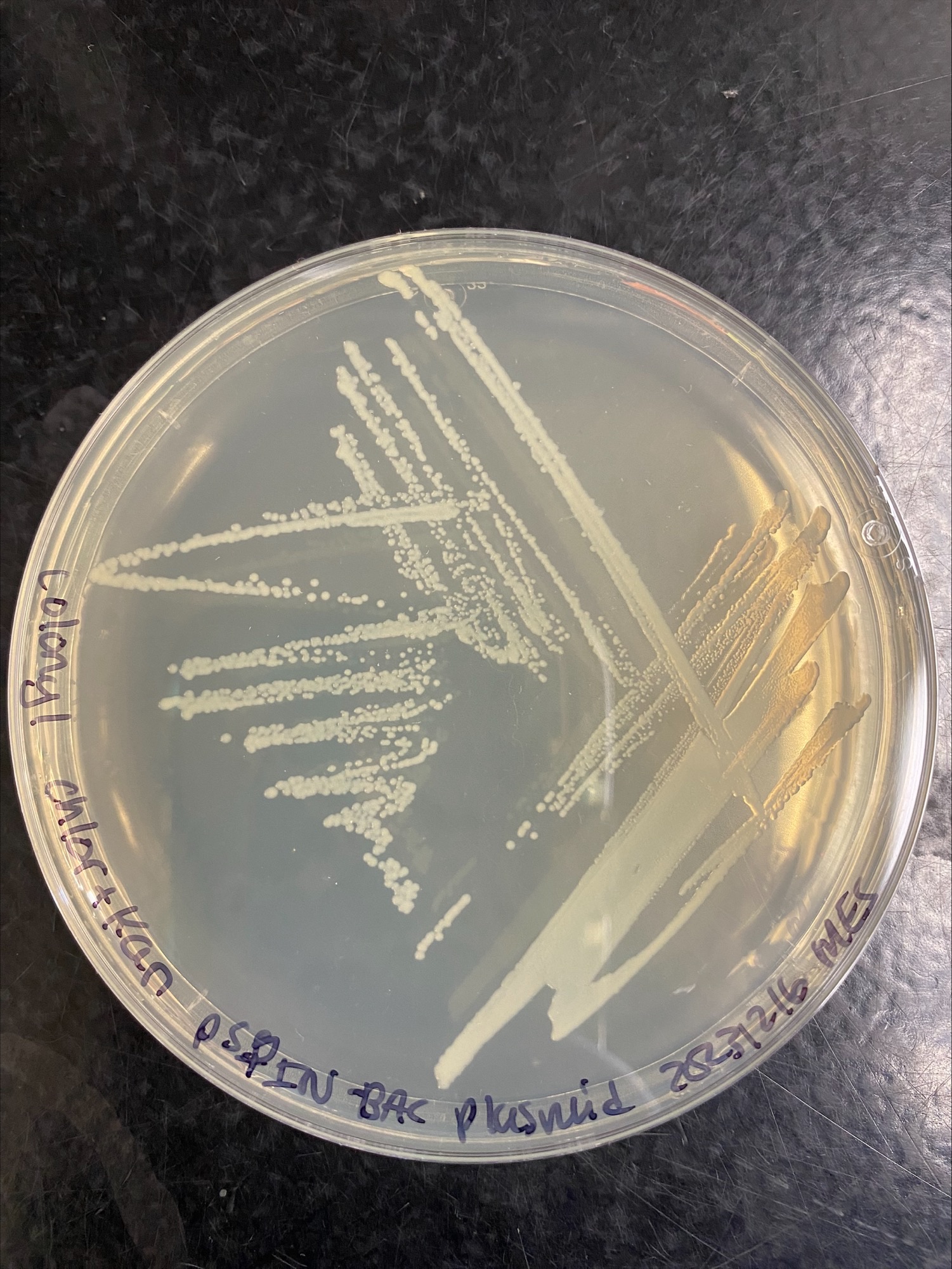
Colony 2 Chloramphenicol:
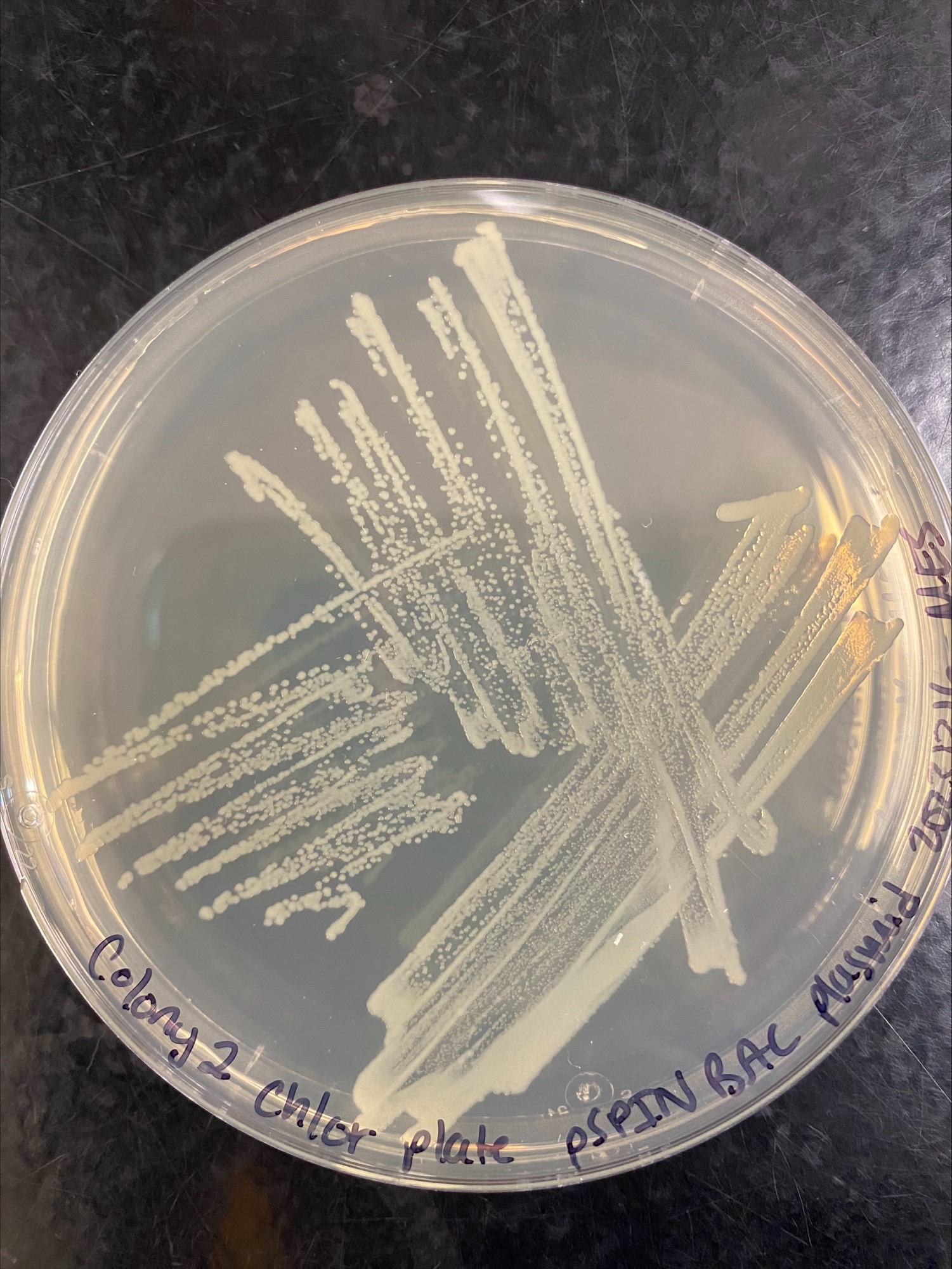 Colony 2 Kanamycin:
Colony 2 Kanamycin:
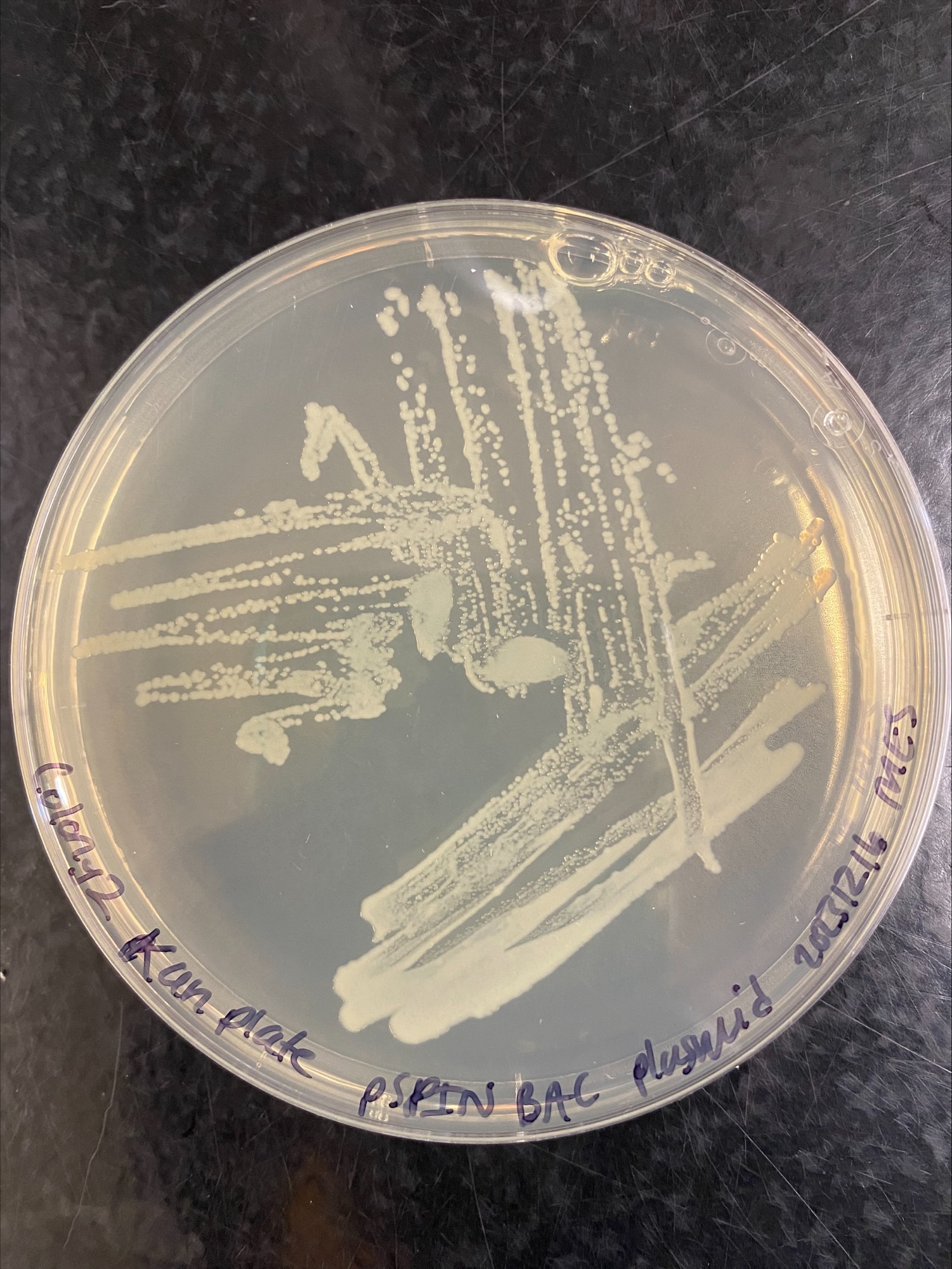 Colony 2 Chloramphenicol and Kanamycin:
Colony 2 Chloramphenicol and Kanamycin:
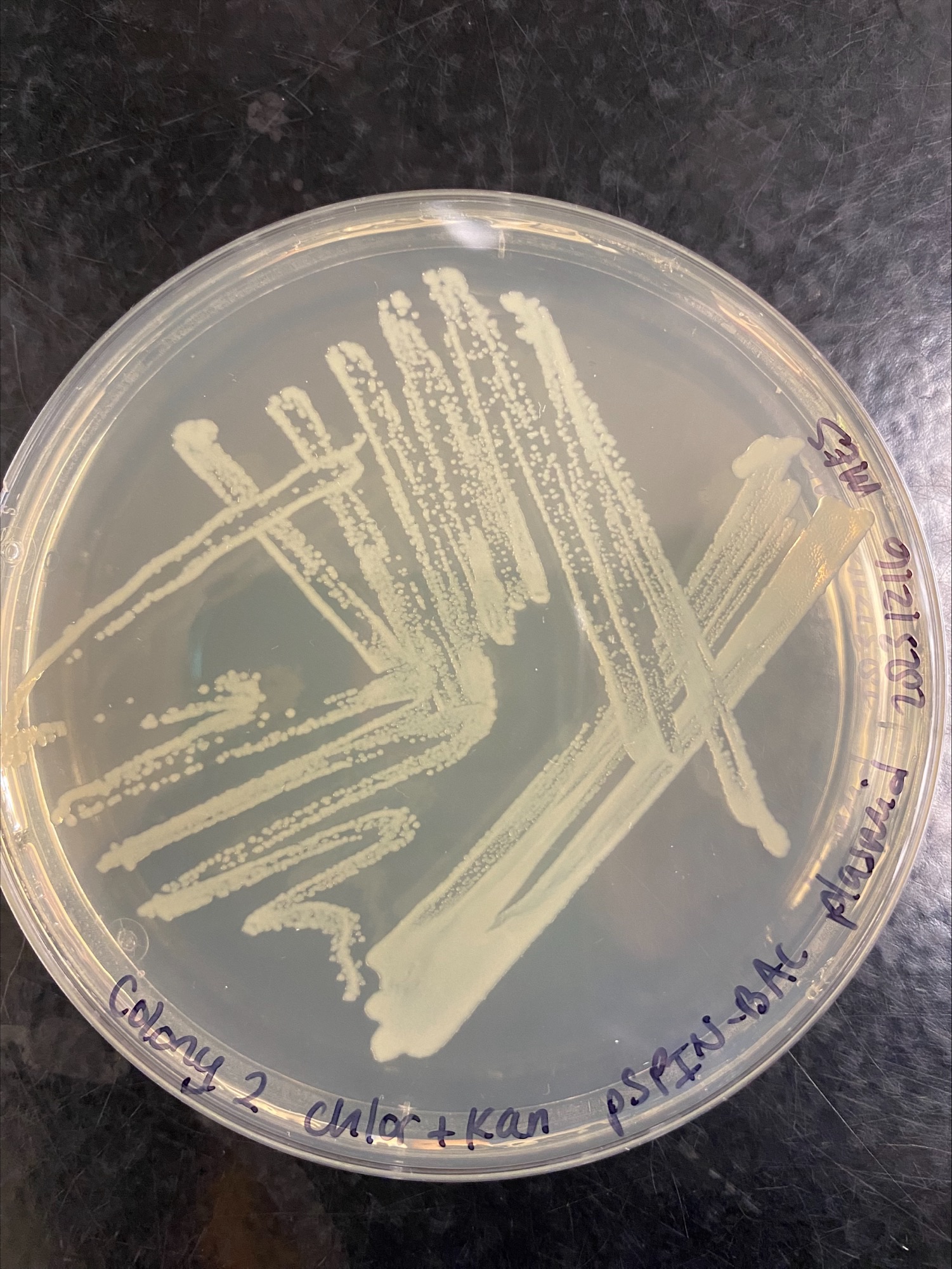
Colony 3 Chloramphenicol:
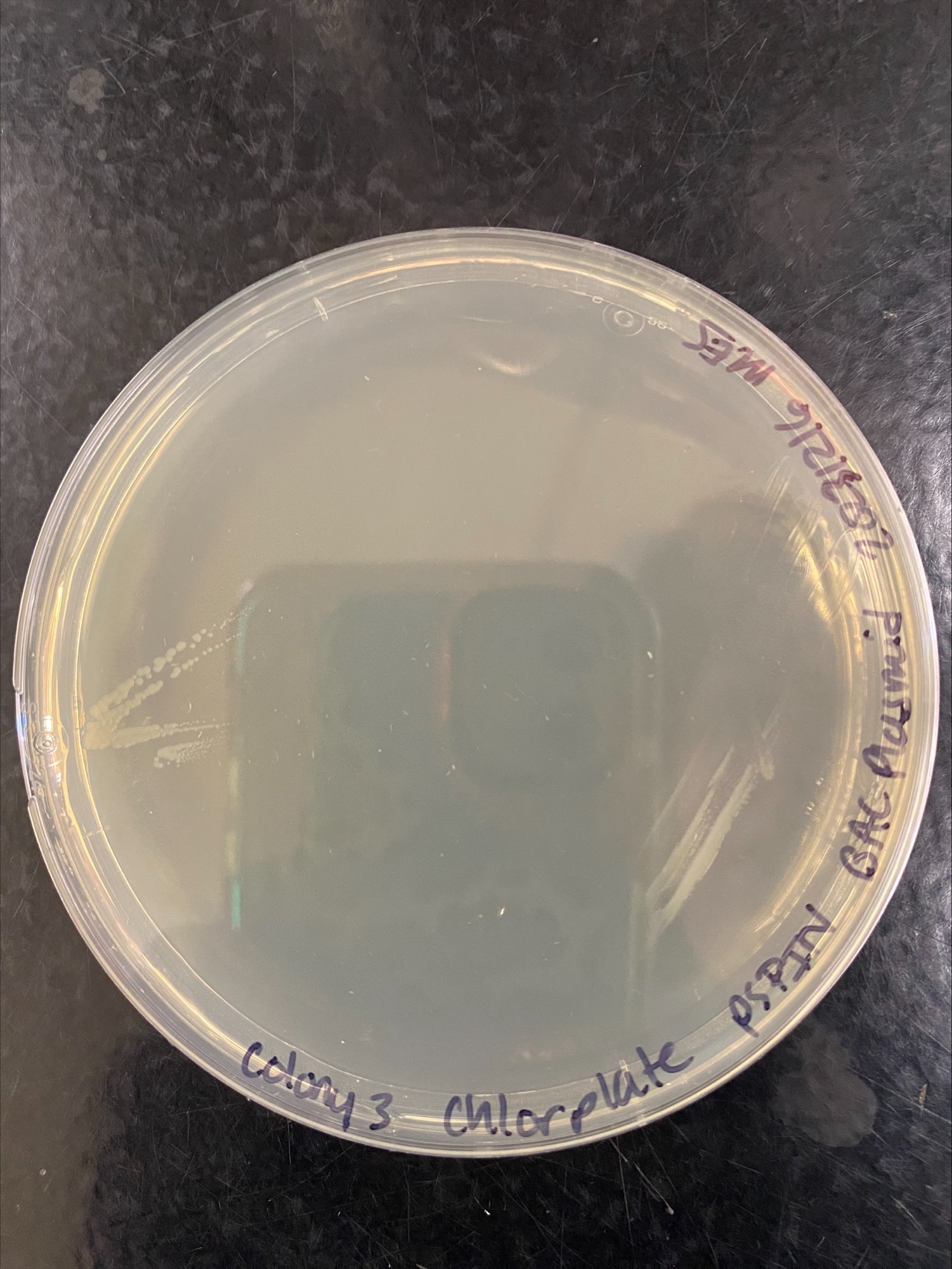 Colony 3 Kanamycin:
Colony 3 Kanamycin:
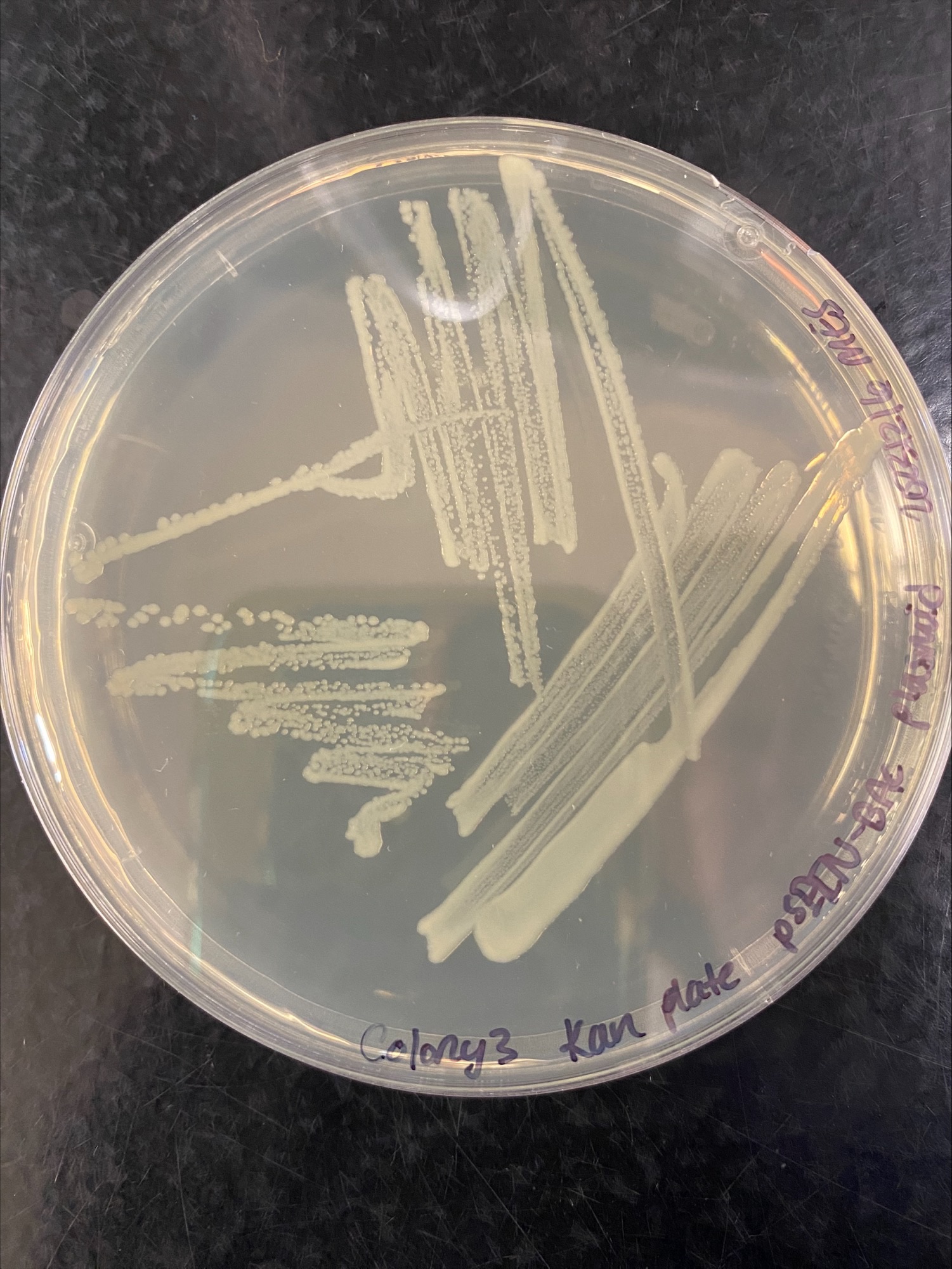 Colony 3 Chloramphenicol and Kanamycin:
Colony 3 Chloramphenicol and Kanamycin:

It looks like colony 3 is not useful to use, and I am glad I tried different colonies for this.