Making Fresh LB Plates and Electroporating the pSPIN-BAC Synthesized by Genewiz into NEB Electrocompetent Cells and Plating on Selective Medium
We had purchased NEB 10-beta electrocompetent cells to use to put the pSPIN-BAC plasmid into. I used the electroporater in the Chandler lab and had the grad student Sam show me how to work it.
20231213 make LB plates
- I wanted to make ~800mL of LB agar
- 12g of bacto agar
- 17.6g Lennox broth
- Add 770mL of water
- All in a 2000mL flask
- Added broth in first, mixed, then added the agar and had spinning on the stir plate
- Autoclaved on setting 3 (use 4 for anything larger than 1L)
- Took agar out and waited not very long to start pouring
- Added 15mL to each plate in a sleeve of plates, using the serological pipettor
- Left on the counter to solidify overnight, then placed upside down in the fridge for storage
From talking with Sam, I planned on using 50ul of cells (what they use in their lab, NEB suggests 25 but it says it can vary), doing a negative control of electroporating cells with no plasmid, and doing 3 dilutions of the cells afterwards for plating. Ones of 10ul, 40ul, then 150ul of bacteria solution. Sam said I should get colonies with the 10ul, but you should always do the others just in case. The steps below are a combination of the NEB protocol and Sam’s suggestions. Because the pSPIN has Kanamycin resistance I decided to use that for the selection plates. It should also be Chloramphenicol resistent to because of the BAC insertion, but I wasn’t sure at the time if it was being expressed.
20231214 Electroporating
- Resuspended dried plasmid (2ug) in 5ul of low EDTA TE
- Flicked to mix it and spun it down a few times, let sit on the bench a which before placing on ice
- SOC medium was allowed to thaw in 4C overnight and will be kept in there
- Prepared 2 1.5mL tubes with 950ul of SOC
- Prepared 1 1.5mL tubes with 500ul LB
- Prepared 6 plates with 50ug/mL Kanamycin
- (100,000mg/mL)(x) = 50ug * 25mL
- 12.5ul of stock Kanamycing per plate
- Because there were 6 plates, and I wanted to add 20ul of liquid to each plate I make a solution of 75ul of stock Kanamycin and 46ul of molecular grade water
- These were pipetted onto the plates and spread with a sterile spreader and allowed to settle in for a few minutes
- Then the plates were kept at 37C until use
- In the Chandler Lab:
- Thawed the electrocompetent cells on ice for ~10 minutes
- Chilled the electroporation cuvettes on ice
- Put the tubes of SOC in their 37C incubator
- Added 50ul of cells to the first cuvette - neg control
- Electroporated on the EC2 settings
- Immediately added 950ul of warm SOC buffer and pipetted to mix
- Transferred the 1000ul into a new 1.5mL tube labeled neg and placed in the 37C incubator
- Added 50ul of cells to the second cuvette - plasmid sample
- Added 2ul of resuspended plasmid to the cuvette
- Pipette mixed gently (Potentially I should have done this in a 1.5mL tube then transferred to the cuvette)
- Electroporated on the EC2 setting
- Immediately added 950ul of warm SOC buffer and pipetted to mix
- Transferred the 1000ul into a new 1.5mL tube labeled neg and placed in the 37C incubator
- Let the tubes shake in the Chandler Lab 37C incubator for 1 hour
- After the 1 hour, I took the tubes down to 4012 (burner on)
- Centrifuged the tubes for 3 min at 3,000rpm
- Removed the supernatant from each tube
- Resuspended each pellet in 200ul of LB
- Did 3 amounts for each sample on the Kanamycin plates, each being spread with a sterile loop spreader (the larger volumes were harder to spread)
| plate | volume | cells |
|---|---|---|
| 1 | 10ul | neg control |
| 2 | 40ul | neg control |
| 3 | 150ul | neg control |
| 4 | 10ul | plasmid |
| 5 | 40ul | plasmid |
| 6 | 150ul | plasmid |
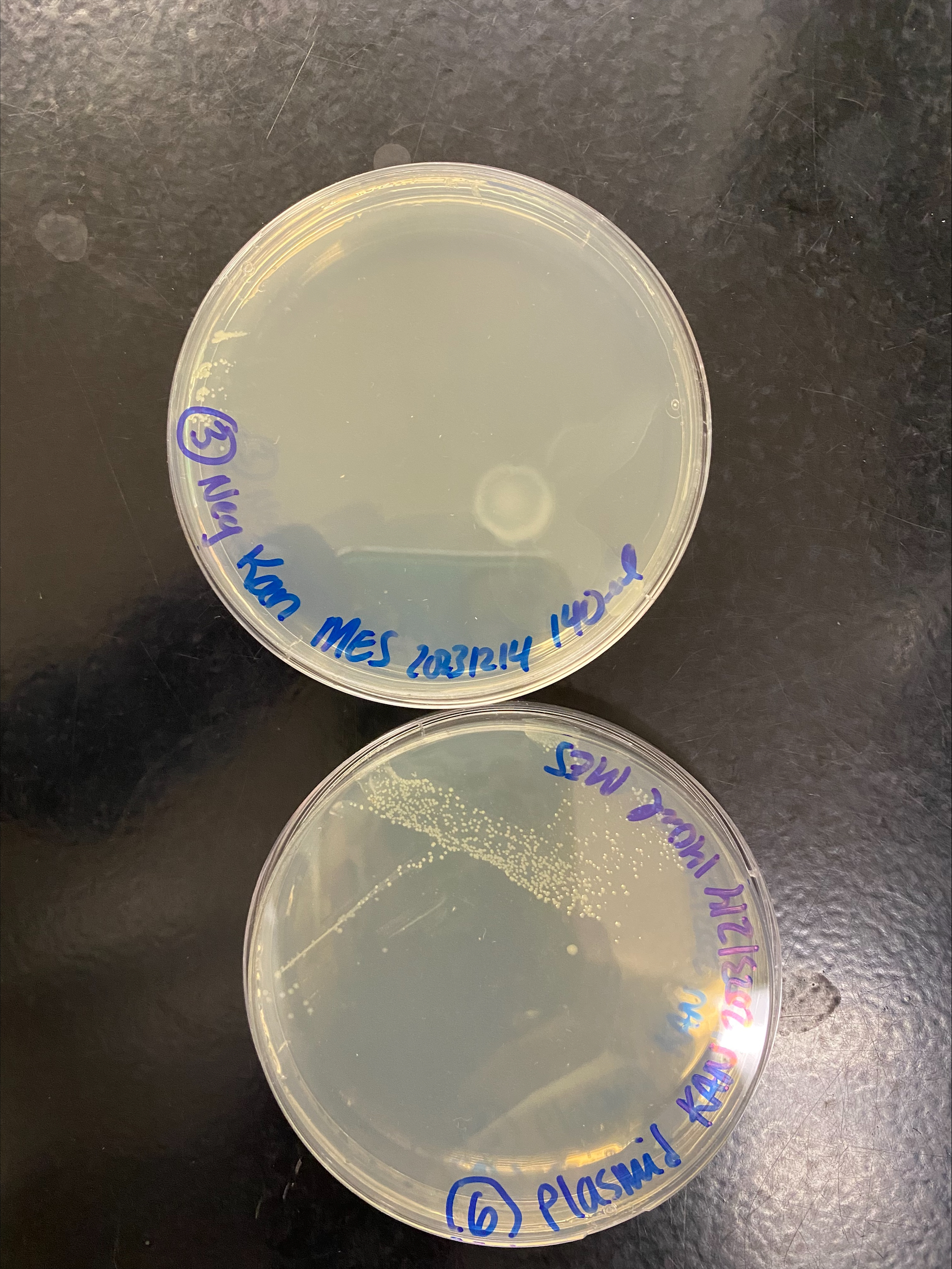
- Placed the plates upside down in the 37C incubator to let them grow overnight
- The next morning I checked on the plates, there was the most growth on the plates that had the plasmid, and the only growth in the middle of the plate where the streaks were. There was a very small amount of growth on the neg control plates, only in the spots where they original bacteria was pipetted where I thought they grew from the non-antibiotic LB
- I let the plates grow for a few more hours and only the plasmid plates showed visible colony growth