Testing 0.5-0.75ul 2020 Transfection Reagent Volumes for Increased GFP in Dv-1 Cells
Using the TransIT®-2020 Transfection Reagent which had the best transfection at 1ul for the previous test, now I want to test less than 1ul transfection reagent volumes. I am not using the other reagent in this test. I will try 0.75ul 2020 reagent and 0.5ul 2020 reagent.
20221209 Plating S2 and DV-1 cells
- I want to use S2 cells again as a control to show that the transfection works
- I need to use the same density I have used previously: 1.5 * 10^5 cells per well in a 12 well plate
- Need to count cells and then separate
- Plate layout:

- This means I will need 3 wells of S2 cells (4.5mL total volume) and 9 wells of Dv-1 cells (13.5mL total volume)
- S2 cells
- I cell scrapped one flask from 11/29 and centrifuged it at 800rpm 2 minutes, removed the supernatant, and resuspended the pellet in 6mL of 10% FBS Schneider’s medium
- 20ul from the resuspention was added to a hemocytometer and counts from each section of the hemocytometer were taken:
| section | quadrant 1 | quadrant 2 | quadrant 3 | quadrant 4 | section average |
|---|---|---|---|---|---|
| 1 | 273 | 265 | 259 | 259 | 264 |
| 2 | 278 | 252 | 276 | 267 | 268 |
- Each section was averaged, and the average of the two sections was taken
- Total S2 average: 266
- The cells per mL is the average * 10^4
- 266 * 10^4 = 2,660,000 cells per mL
- Now I need to dilute this so that I add ~1.5 * 10^5 cells to each well in 1.5mL aliquoits
- I used M1V1=M2V2 to determine how much volume would be needed to dilute the entire concentrated liquid, and then scaled down for the volume I actually needed
- (2.66 * 10^6 cells/mL)*(5mL) = (1.5 * 10^5 cells)(x mL)
- x = 89mL
- I don’t need this much volume, so I divided the original volume (5mL) and dilution volume by 5
- Added 16.8mL medium to a new tube
- Used 1mL of the concentrated cell suspension to that tube
- These were mixed and added to the A1, B1, and C1 wells of PLATE 7
- The rest of the diluted S2 cells were seeded into new flasks
- Dv-1
- I cell scrapped 1 Dv-1 cell flask, centrifuged them for 3 minutes at 800rpm, and removed the liquid. Then the cell pellet was resuspended in 5mL of 10% FBS Schneider’s medium
- 20ul from the resuspention was added to a hemocytometer and counts from each section of the hemocytometer were taken:
| section | quadrant 1 | quadrant 2 | quadrant 3 | quadrant 4 | section average |
|---|---|---|---|---|---|
| 1 | 310 | 365 | 336 | 294 | 326 |
| 2 | 377 | 357 | 286 | 344 | 316 |
- Each section was averaged, and the average of the two sections was taken
- Total Dv-1 average: 321
- The cells per mL is the average * 10^4
- 321 * 10^4 = 3,210,000 cells per mL
- I used M1V1=M2V2 to determine how much volume would be needed to dilute the entire concentrated liquid, and then scaled down for the volume I actually needed
- (3.21 * 10^6 cells/mL)*(5mL) = (1.5 * 10^5 cells)(x mL)
- x = 107mL
- I don’t need this much volume, so I divided the original volume (5mL) and dilution volume by 4
- Added 25.5mL medium to a new tube
- Used 1.25mL of the concentrated cell suspension to that tube
- These were mixed and added to wells A2, B2, C2, A3, B3, C3, A4, B4, and C4
- The rest of the diluted Dv-1 cells were seeded into new flasks
- The plate was left to grow overnight in the 23 degree incubator
20221208 Transfection
- I am using the same concentration of the pAc5 plasmid as I used previously
- I checked the densities of the cells before doing the transfections. There seems to be similar amounts of cells in each plate, maybe a few more Dv-1 cells even though I tried to make them the same
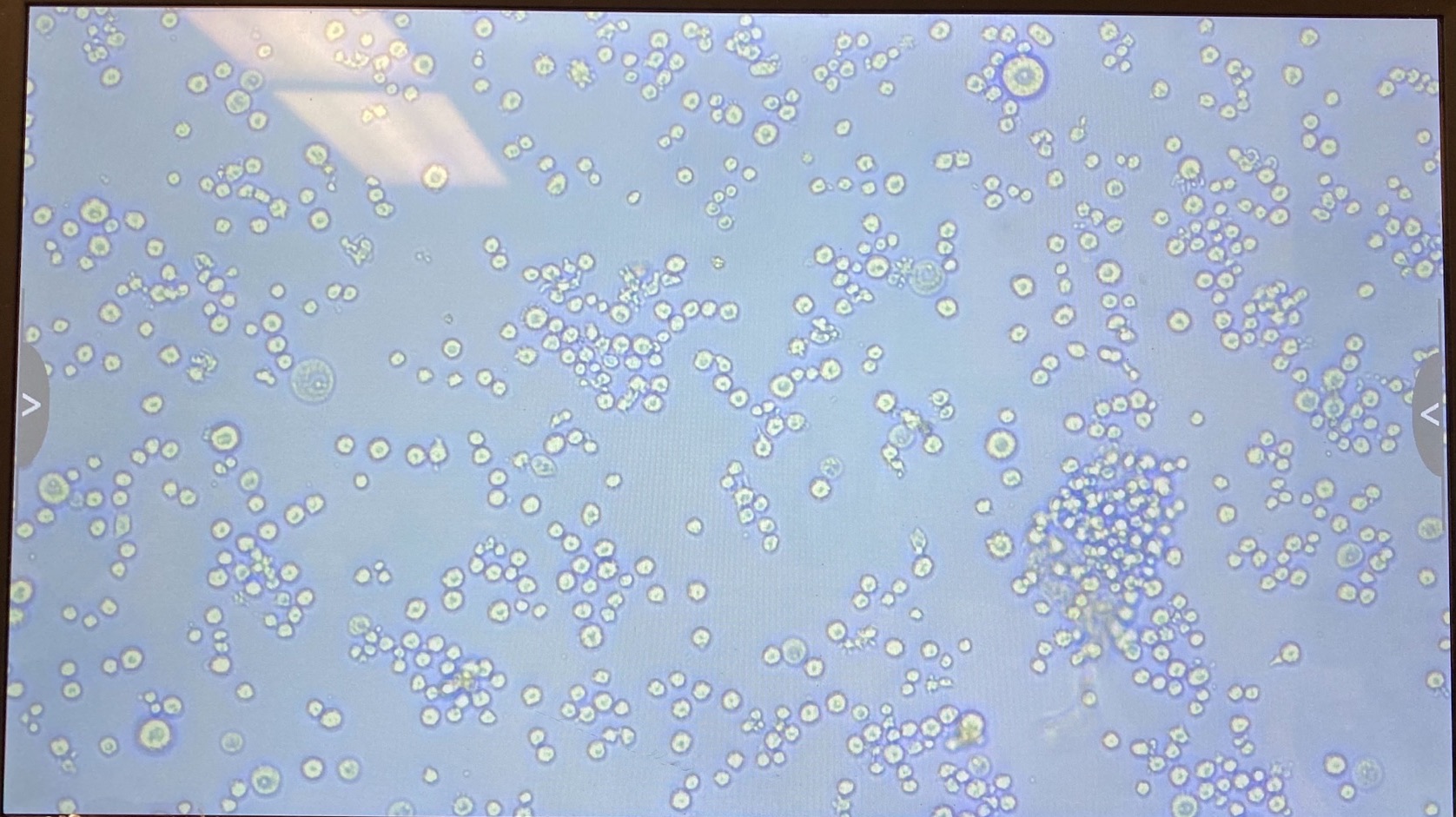
- S2
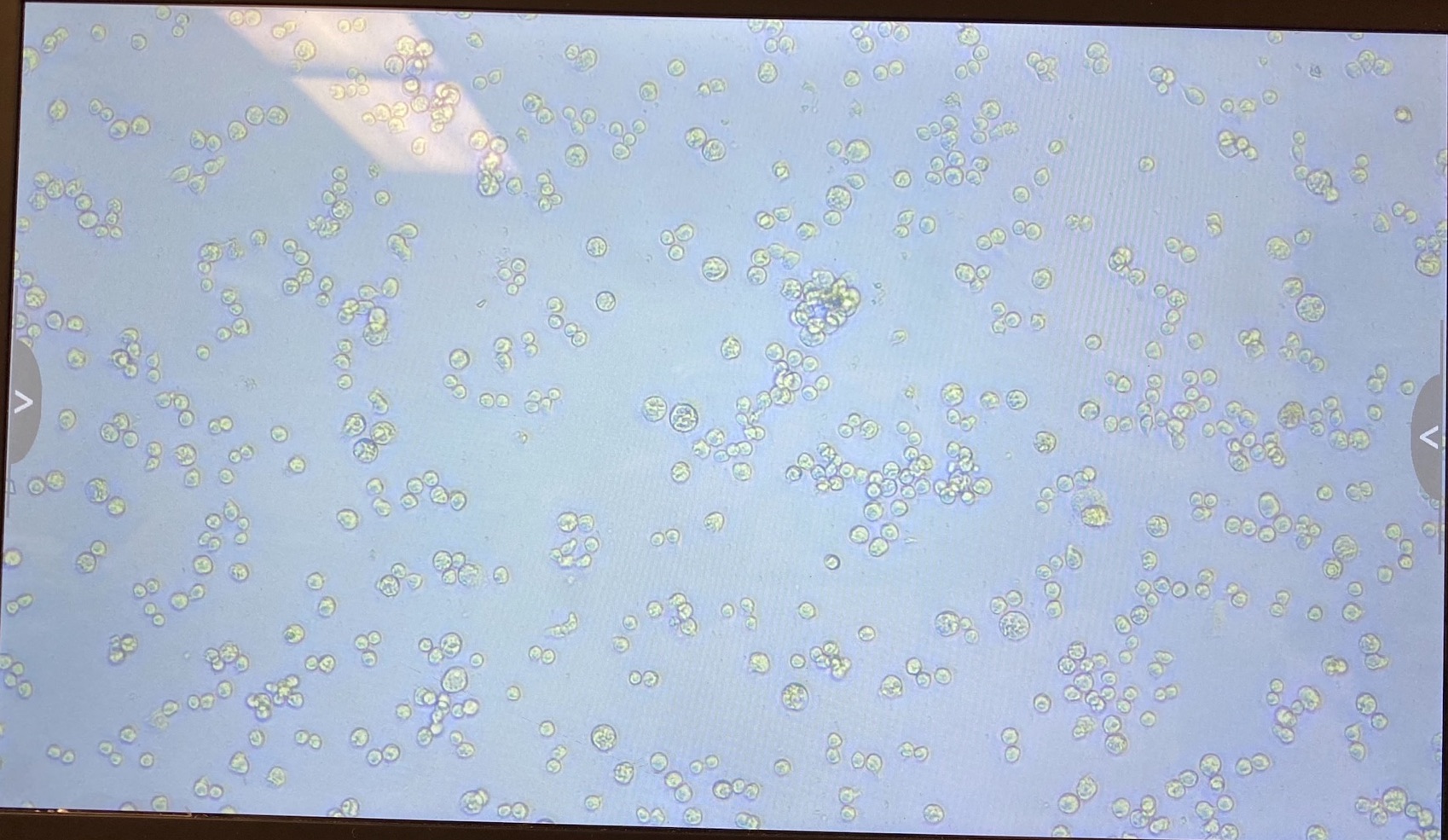
- Dv-1
- 2020 reagent was thawed at room temp and vortexed and spun down, so was the plasmid
- All work was done in the cell culture hood
- All reaction mixes were made up beforehand:
- Tube 1
- 400ul Schneider’s medium
- Tube 2
- 300ul Schneider’s medium
- 3ul concentrated pAc5 plasmid
- 3ul 2020 transfection reagent
- Tube 3
- 100ul Schneider’s medium
- 1ul concentrated pAc5 plasmid
- 3ul 2020 transfection reagent
- Tube 4
- 200ul Schneider’s medium
- 2ul concentrated pAc5 plasmid
- 1.5ul 2020 transfection reagent
- Tube 5
- 200ul Schneider’s medium
- 1ul concentrated pAc5 plasmid
- 1ul 2020 transfection reagent
- All reaction tubes were pipette mixed genetly
- Let tubes incubate in the hood for 30 minutes
- After the 30 minute incubation, all experimental reagents were added dropwise to the appropiate wells
- 100ul tube 1 to wells A1, A2, A3, and A4
- 102ul tube 2 to wells B1, C2, and C2
- 104ul tube 3 to well C1
- 101.75ul tube 4 to wells B3 and C3
- 101.5ul tube 5 to wells B4 and C4
- Plates were gently rocked back and forth and placed in the 23 degree incubator to be checked every 24 hours for a few days
The plate was imaged ~every 24 hours with a scope capable of visualizing GFP. Images can be found here