Testing Transfection Reagent Volumes for Increased GFP in Dv-1 Cells
With the recomended amounts of TransIT®-Insect Transfection Reagent](https://www.mirusbio.com/products/transfection/transit-insect-transfection-reagent) and the TransIT®-2020 Transfection Reagent for Dv-1 cells there was only a mild expression of GFP. The protocols say I can use 1-4ul of reagent per 1ug of DNA, so I will be testing the volumes of reagent I haven’t previously used.
20221129 Plating S2 and DV-1 cells
- I want to use S2 cells again as a control to show that the transfection works
- I want to plate these at a density of 0.8-3 * 10^5 cells as per the instructions for the transfection reagents. I have been doing 1.5 * 10^5 and it has been working well
- Need to count cells and then separate
- Plate layouts:

- This means I will need 3 wells of S2 cells (4.5mL total volume) and 18 wells of Dv-1 cells (27mL total volume)
- S2 cells
- I cell scrapped one flask from 11/22 and centrifuged it at 800rpm 2 minutes, removed the supernatant, and resuspended the pellet in 6mL of 10% FBS Schneider’s medium
- 20ul from the resuspention was added to a hemocytometer and counts from each section of the hemocytometer were taken:
| section | quadrant 1 | quadrant 2 | quadrant 3 | quadrant 4 | section average |
|---|---|---|---|---|---|
| 1 | 310 | 235 | 262 | 382 | 297 |
| 2 | 287 | 378 | 330 | 339 | 333 |
- Each section was averaged, and the average of the two sections was taken
- Total S2 average: 315
- The cells per mL is the average * 10^4
- 315 * 10^4 = 3,150,000 cells per mL
- Now I need to dilute this so that I add ~1.5 * 10^5 cells to each well in 1.5mL aliquoits
- I used M1V1=M2V2 to determine how much volume would be needed to dilute the entire concentrated liquid, and then scaled down for the volume I actually needed
- (3.15 * 10^6 cells/mL)*(6mL) = (1.5 * 10^5 cells)(x mL)
- x = 105mL
- I don’t need this much volume, so I divided the original volume (6mL) and dilution volume by 7
- Added 14.1mL medium to a new tube
- Used 0.86mL of the concentrated cell suspension to that tube
- These were mixed and added to the A1, B1, and C1 wells of PLATE 5
- Dv-1
- I cell scrapped 1 Dv-1 cell flask, centrifuged them for 3 minutes at 800rpm, and removed the liquid. Then the cell pellet was resuspended in 5mL of 10% FBS Schneider’s medium
- 20ul from the resuspention was added to a hemocytometer and counts from each section of the hemocytometer were taken:
| section | quadrant 1 | quadrant 2 | quadrant 3 | quadrant 4 | section average |
|---|---|---|---|---|---|
| 1 | 348 | 356 | 343 | 365 | 353 |
| 2 | 261 | 284 | 339 | 304 | 297 |
- Each section was averaged, and the average of the two sections was taken
- Total Dv-1 average: 325
- The cells per mL is the average * 10^4
- 325 * 10^4 = 3,250,000 cells per mL
- I used M1V1=M2V2 to determine how much volume would be needed to dilute the entire concentrated liquid, and then scaled down for the volume I actually needed
- (3.25 * 10^6 cells/mL)*(5mL) = (1.5 * 10^5 cells)(x mL)
- x = 108.3mL
- I don’t need this much volume, so I divided the original volume (5mL) and dilution volume by 3
- Added 36mL medium to a new tube
- Used 1.7mL of the concentrated cell suspension to that tube
- These were mixed and added to wells:
- PLATE 5: A2, B2, C2, A3, B3, C3, A4, B4, C4
- PLATE 6: A1, B1, C1, A2, B2, C2, A3, B3, C3
- The plate was left to grow overnight in the 23 degree incubator
20221130 Transfection
- First I concentrated another extraction of the pAc5 plasmid to be ~1ug/ul, and added it to the previous concentration so that I would use a homogenous mixture of plasmid
- The total volume is 124ul
- Tube 3 is 158ng/ul
- 158ng/ul in 124ul is 19,592ng total
- I bead cleaned the sample and resuspended it in 19ul of 10mM tris HCl
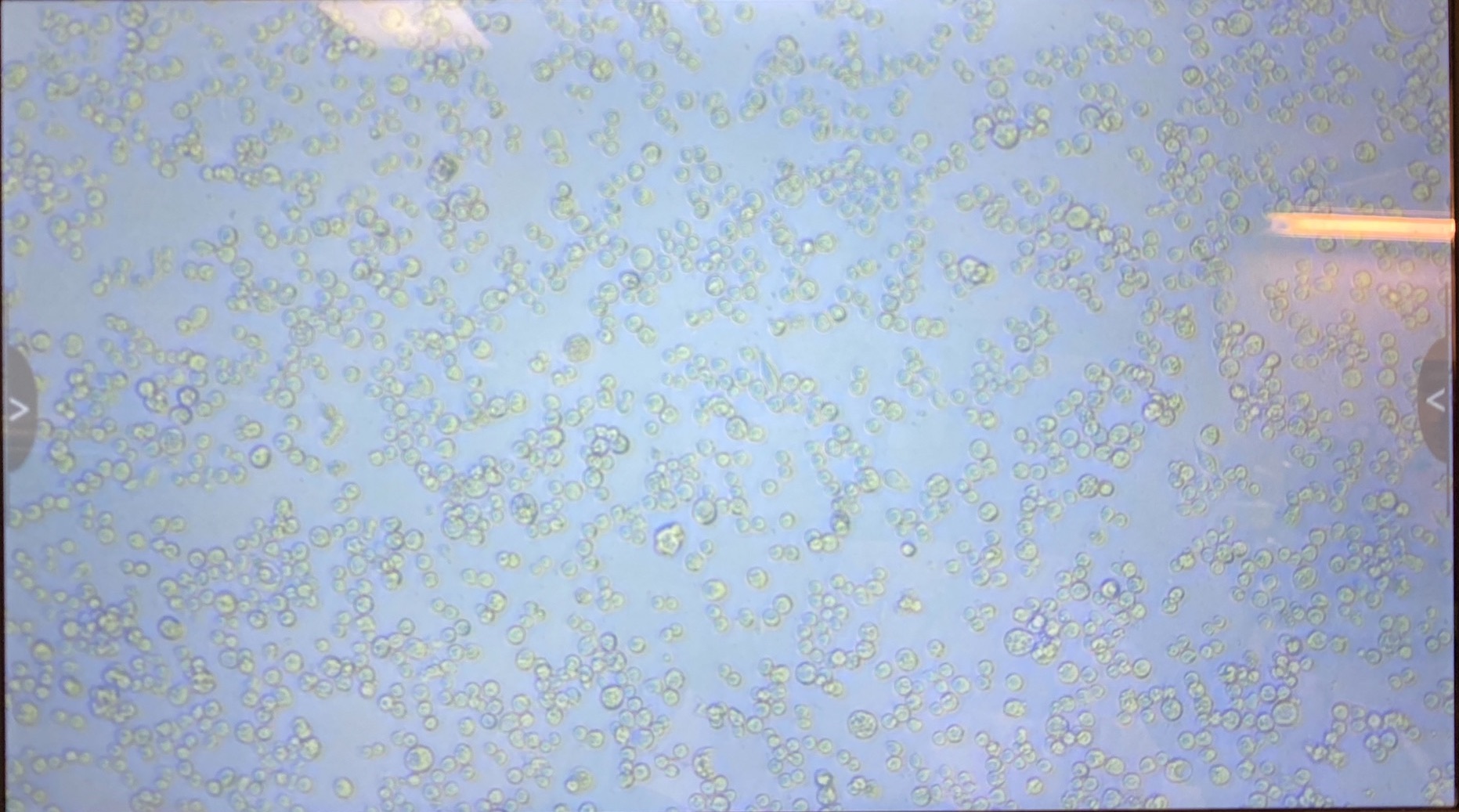
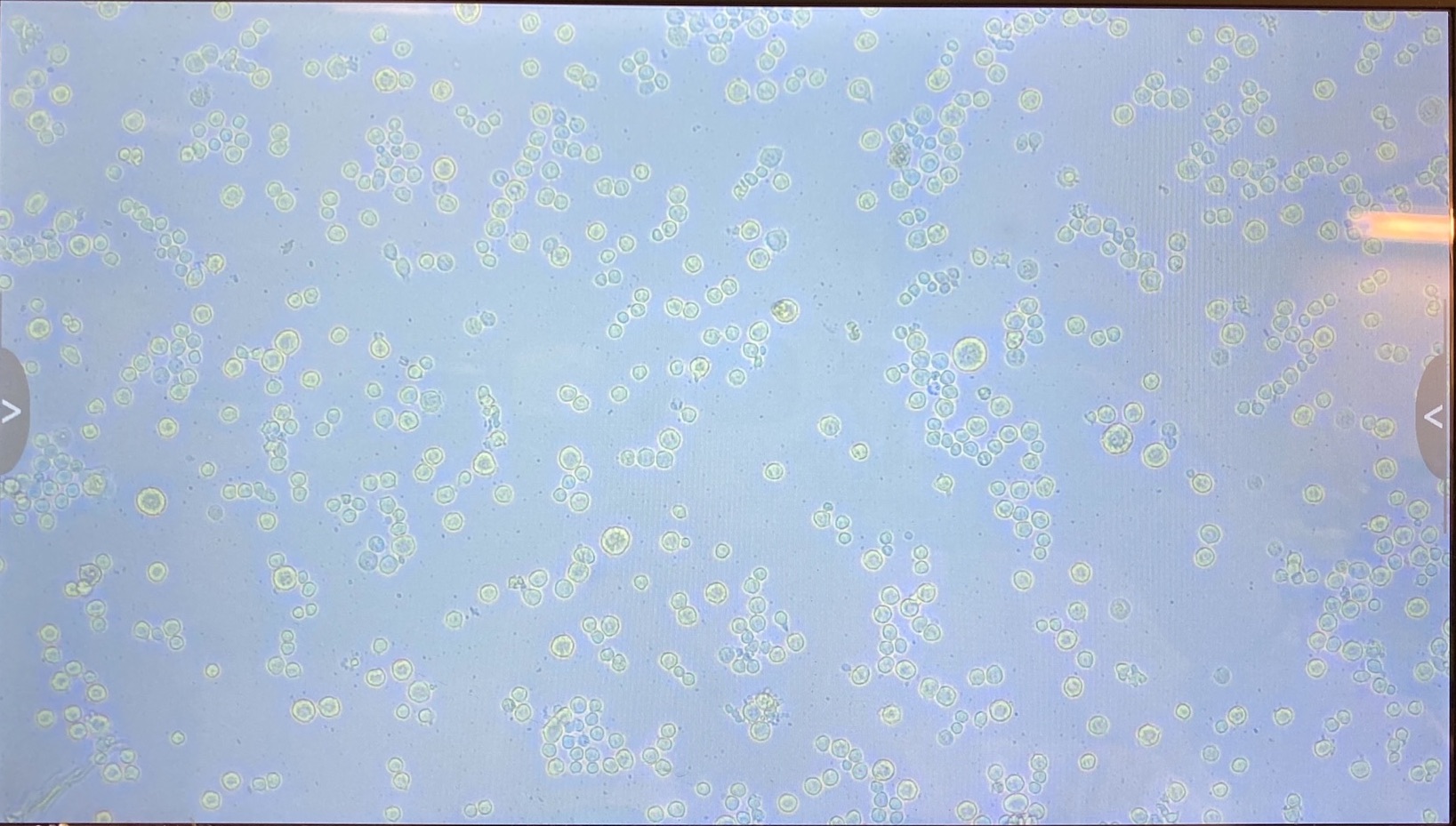
- I checked the densities of the cells before doing the transfections. There seem to be a few more cells in the S2 dishes than the Dv-1 dishes even though I tried to make them standardized
- S2

- Dv-1

- Insect and 2020 reagents were thawed at room temp and vortexed and spun down
- All work was done in the cell culture hood
- All reaction mixes were made up beforehand:
- Tube 1
- 700ul Schneider’s medium
- Tube 2
- 100ul Schneider’s medium
- 1ul concentrated pAc5 plasmid
- 2ul insect transfection reagent
- Tube 3
- 100ul Schneider’s medium
- 1ul concentrated pAc5 plasmid
- 3ul 2020 transfection reagent
- Tube 4
- 200ul Schneider’s medium
- 2ul concentrated pAc5 plasmid
- 2ul insect transfection reagent
- Tube 5
- 200ul Schneider’s medium
- 1ul concentrated pAc5 plasmid
- 6ul insect transfection reagent
- Tube 6
- 200ul Schneider’s medium
- 1ul concentrated pAc5 plasmid
- 8ul insect transfection reagent
- Tube 7
- 200ul Schneider’s medium
- 1ul concentrated pAc5 plasmid
- 2ul 2020 transfection reagent
- Tube 8
- 200ul Schneider’s medium
- 1ul concentrated pAc5 plasmid
- 4ul 2020 transfection reagent
- Tube 8
- 200ul Schneider’s medium
- 1ul concentrated pAc5 plasmid
- 8ul 2020 transfection reagent
- All reaction tubes were pipette mixed genetly
- Let tubes incubate in the hood for 30 minutes
- After the 30 minute incubation, all experimental reagents were added dropwise to the appropiate wells
- 100ul tube 1 to P5 wells A1, A2, A3, A4, and P6 A1, A2, and A3
- 103ul tube 2 to P5 B1
- 104ul tube 3 to P5 C1
- 102ul tube 4 to P5 B2 and P5 C2
- 104ul tube 5 to P5 B3 and P5 C3
- 105ul tube 6 to P5 B4 and P5 C4
- 102ul tube 7 to P6 B1 and P6 C1
- 103ul tube 8 to P6 B2 and P6 B2
- 105ul tube 9 to P6 B3 and P6 B3
- Plates were gently rocked back and forth and placed in the 23 degree incubator to be checked every 24 hours for a few days
The plate was imaged ~every 24 hours with a scope capable of visualizing GFP. Images can be found here