Testing Loading and Running a Gel for HMW DNA using the NEB MidRangePGF Marker, Trying to Find the Optimal Time Running: 15 and 22 Hours
Notes
- First attempt at running this gel was for 5 hours and was no where near long enough
- Talking with Kistie, we decided that I could run it overnight and into the next day. And that I could load ladders on each side of the gel, then after 15 hours I could pause the run, slice out half the gel to image it, and let the other half go for another 5 hours
- I also found a gel comb with larger wells that fit the entire rounds perfectly with no splitting
- I also decided to use 16ul of 48kb ladder as a reference (the amount the protocol suggests using)
Making and Loading the gel, 20211104
- 0.7% gel mix:
- 180mL 1X TAE
- 1.26g agarose
- Microwaved for ~4min
- Pipetted 1mL into a 1.5mL tube and saved on the heat block at 65 degrees C make sure the heat block is on and running
- Made sure the large tray had well sealed tape barriers
- Let the gel liquid cool for ~10 min until pouring into the tray and placing in the combs
- Gel cooled in ~20 min or less
- Added 1 round of PFG ladder to the right and left most wells
- Let the 1mL saved gel cool for ~3 min outside of the heat block
- Filled the PFG wells with cooled gel liquid to the top of the wells to seal the rounds in
- Waited ~3 minutes for the wells to cool
- Made up 48kb ladder:
- 1.2ul ladder
- 6ul loading dye
- 28.8ul molecular grade water
- Placed the gel tray in the gel box (without tape)
- Added 16ul 48kb ladder to the wells right next to the PGF wells
- Added 5ul 1kb plus ladder to the wells right next to the 48kb wells
- Filled the box to the MAX level with 1X TAE
- Set the timer for 16 hours at 46 volts and started it at 5:36pm
Imaging the gels 20211105
- Stopped the gel at 8:42am (a little over 15 hours)
- Took out the gel tray and put it on the counter
- Sliced the gel down the middle with a razor blade
- Slid off the right half of the gel very carefully (fragile!) and placed in the EtBr bath for 1 hour
- Placed the tray back into the box and started it again for ~5 more hours (at 8:48am)
- After incubation in the EtBr, the gel was imaged:
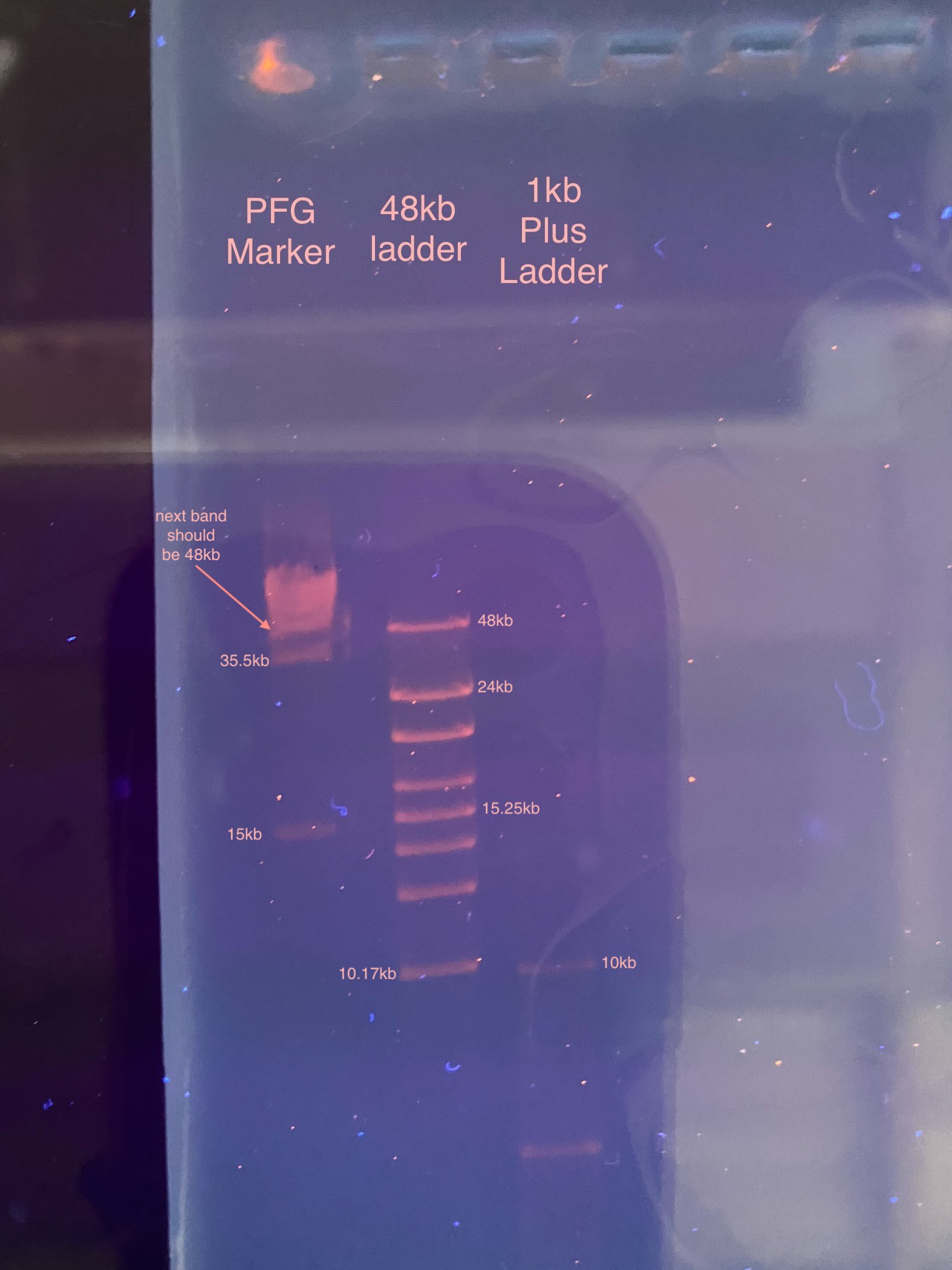
- After seeing how little everything ran, I decided to up the time running the gel until 4pm (~22.5 hours)
- At 4pm I stopped the gel, slid the left half into the EtBr, and let it incubate for 1 hour
- After incubation in the EtBr, the gel was imaged:

Clearly the voltage is too low, or the agarose % is too high, to get good band resolution on the PFG marker. This was pretty much the maximum amount of time to run this probably, it looks like the longer the gel is run the lest bright the bands are. They all had the same amount of incubation time in the EtBr, so something must be happening to decrease the amount of DNA.