RNA Extraction of Pentagona and Hystera Sea Stars, 6 Samples
Notes
- Using the Zymo DNA/RNA Miniprep Plus kit
- Used 1.4mm ceramic beads and the tissuelyser for 2 minutes at 25Hz, stopped at 30 seconds and moved the tubes to the other side of the sample holder in the lyser.
- Incubated at room temp with the proteinase K for 15 minutes shaking at 550rpm
Sample Preparation
- Samples:
- CHSB-002
- CHSB-003
- CHSY-003
- CPAB2-005
- CPAB2-009
- CPOI-008
- Thawed samples on ice bucket
- Prepared forceps, foil, and razor blades
- Cleaned forceps with 10% bleach, DI water, 70% ethanol, and RNaseZap before and between each sample
- Made 1 ceramic bead tube with 800ul DNA/RNA shield for each tissuelyser sample
- Cut a small piece of tissue for each sample then minced with the razor blade until it was flat and could come apart a little bit and placed in their respective bead homogenization tube
- Ran the tissuelyser for 2 minutes at 25Hz
- Spun down the bead tubes in the minifuge to remove bubbles
- Added 80ul proteinase K digestion buffer and 40ul proteinase K to each bead tube
- Briefly vortexed and spun down sample tubes
- Placed in the thermomixer for 15 minutes shaking at 550rpm at 23 degrees C temp (room temp)
- After homogenization and incubation CHSB-002:
- CHSB-003
- CHSY-003
- CPAB2-005
- CPAB2-009
- CPOI-008
- Removed all the supernatant (~800ul) into new 1.5mL tubes
- Centrifuged tubes for 1 minute at 16,00rcf
- Removed liquid (~800ul) into new 1.5mL tubes avoiding small pellet
- Added equal volume (800ul) DNA/RNA lysis buffer to the 5mL tubes
- Vortexed and spun down tubes
- Flicked and inverted tubes to mix and spun down
- Warmed 10mM Tris HCl pH 8 in the thermomixer at 70 degrees C note did not warm H2O this time
- Added 700ul of the liquid to yellow spin columns and collection tubes
- Centrifuged 16,000rcf for 30 seconds
- Saved the flow through in new 5mL tubes
- Repeated addition, centrifugation, and saving of flowthrough for the remaining amount of liquid (3 total spins)
- Added 400ul DNA/RNA prep buffer to the yellow spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 700ul DNA/RNA wash buffer to the yellow spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 400ul DNA/RNA wash buffer to the yellow spin columns
- Centrifuged 16,000rcf for 2 minutes
- Transferred spin columns to new 1.5mL tubes for each sample labeled as final DNA tubes
- Discarded flow through and collection tubes
- Added 50ul warmed 10mM Tris HCl to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Added another 50ul warmed 10mM Tris HCl to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Discarded the spin columns
- Made strip tubes with 7ul of each sample in them for QC
- Kept tubes on ice bucket then stored in -20 degree freezer
- Added equal volume (1600ul) 100% ethanol to the 5mL tubes of flowthrough
- Vortexed and spun down
- Added 700ul of the liquid to green spin columns and collection tubes
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Repeated addition, centrifugation, and saving of flowthrough for the remaining amount of liquid (5 total spins)
- Added 400ul DNA/RNA wash buffer to the green spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Created the DNase mix:
- 75ul DNA digestion buffer * 6 = 450ul
- 5ul DNase I * 6 = 30ul
- Flicked and spun down mix
- Added 80ul DNAse mix to each green spin column filter
- Incubated for 15 minutes at room temp
- Added 400ul DNA/RNA prep buffer to the green spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 700ul DNA/RNA wash buffer to the green spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 400ul DNA/RNA wash buffer to the green spin columns
- Centrifuged 16,000rcf for 2 minutes
- Transferred spin columns to new 1.5mL tubes for each sample labeled as final RNA tubes
- Discarded flow through and collection tubes
- Added 50ul room temp nuclease free water to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Added another 50ul room temp nuclease free water to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Discarded the spin columns
- Made strip tubes with 5ul of each sample in them for QC
- Kept tubes on ice bucket then stored in -80 degree freezer
QC
Broad Range Qubit for DNA and RNA
| Sample |
Reading 1 (ng/ul) |
Reading 2 (ng/ul) |
Average DNA (ng/ul) |
| Standard 1 |
187 RFU |
- |
- |
| Standard 2 |
20207 RFU |
- |
- |
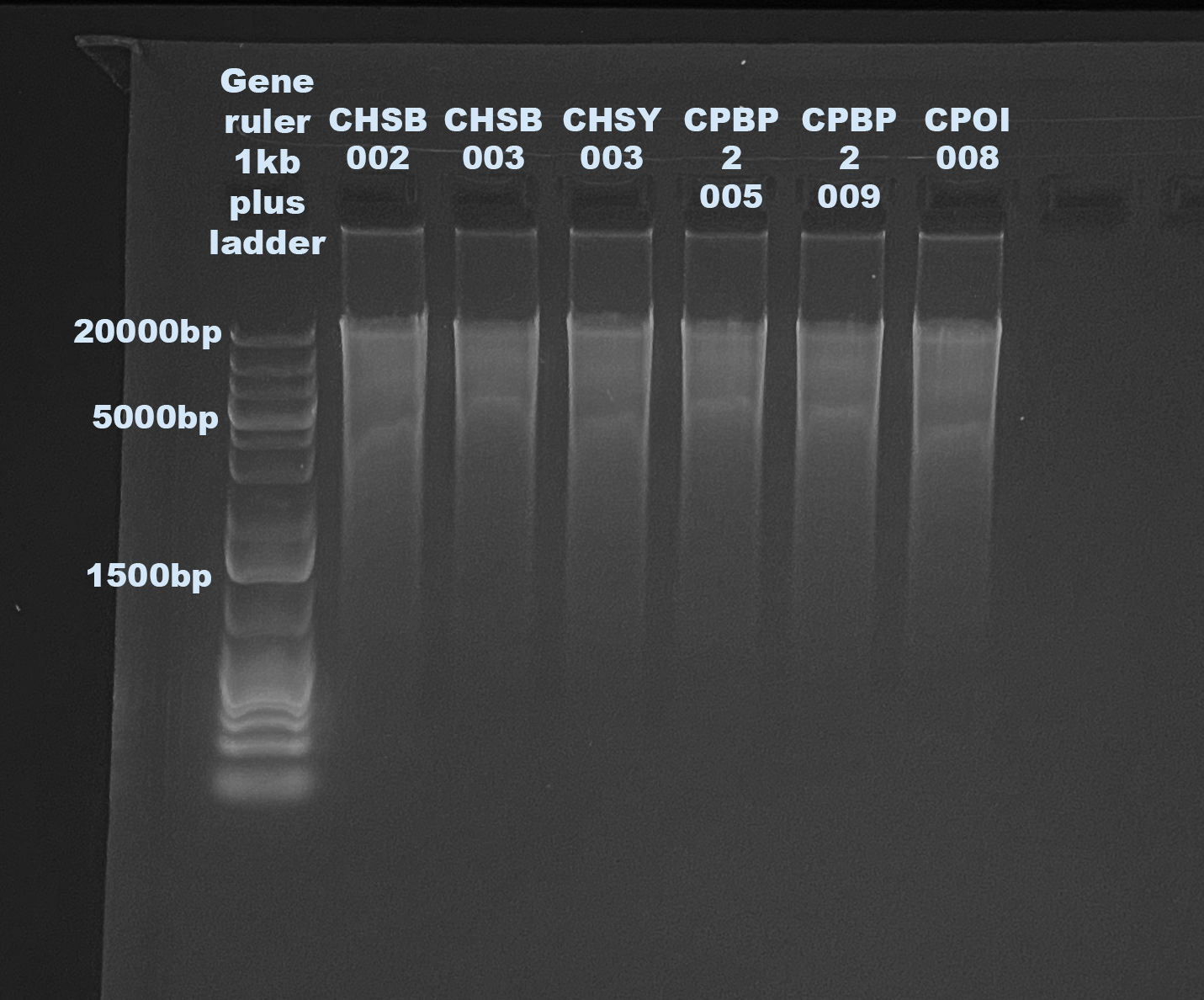
| CHSB-002 |
45.8 |
45.6 |
45.5 |
| CHSB-003 |
22.8 |
22.6 |
22.5 |
| CHSY-003 |
34.6 |
34.2 |
34.4 |
| CPAB2-005 |
21.6 |
21.6 |
21.6 |
| CPAB2-009 |
26 |
25.4 |
25.7 |
| CPOI-008 |
37 |
36.6 |
36.8 |
| Sample |
Reading 1 (ng/ul) |
Reading 2 (ng/ul) |
Average RNA (ng/ul) |
| Standard 1 |
389 RFU |
- |
- |
| Standard 2 |
8413 RFU |
- |
- |
| CHSB-002 |
19.2 |
19 |
19.1 |
| CHSB-003 |
11 |
10.8 |
10.9 |
| CHSY-003 |
11 |
10.8 |
10.9 |
| CPAB2-005 |
too low |
- |
- |
| CPAB2-009 |
too low |
- |
- |
| CPOI-008 |
12 |
11.4 |
11.7 |
1% Agarose Gel for DNA Quality
RNA TapeStation
- Followed RNA tapestation protocol
- TapeStation Report link
- I ended up tapestationing all the samples because I thought there was some RNA in the CPAB2 samples, it could just be too low for the Qubit to read. Turns out I was right. I also looked up the Quant-IT RNA assay guide and it’s not supposed to quantify below 5ng (that’s 5ng/ul), but this kit is made to use in the plate reader, so my guess is that it’s a little off using the Qubit and can’t read below 10ng/ul
Written on November 18, 2020