Troubleshooting DNA/RNA Extraction of Pentagona and Hystera Sea Stars 6
DNA/RNA Extractions on 3 Sea Star Tissue Samples Stored in DNA/RNA Shield, 2 Pentagona and 1 Hystera, Using the Tissuelyser for 1 Minute at 15Hz and Incubating at Room Temperature for 15 Minutes
Notes
- Using the Zymo DNA/RNA Miniprep Plus kit
- Used 1.4mm ceramic beads and the tissuelyser for 1 minute at 15Hz, stopped at 30 seconds and moved the tubes to the other side of the sample holder in the lyser.
- Incubated at room temp with the proteinase K for 15 minutes shaking at 550rpm
Sample Preparation
- Samples:
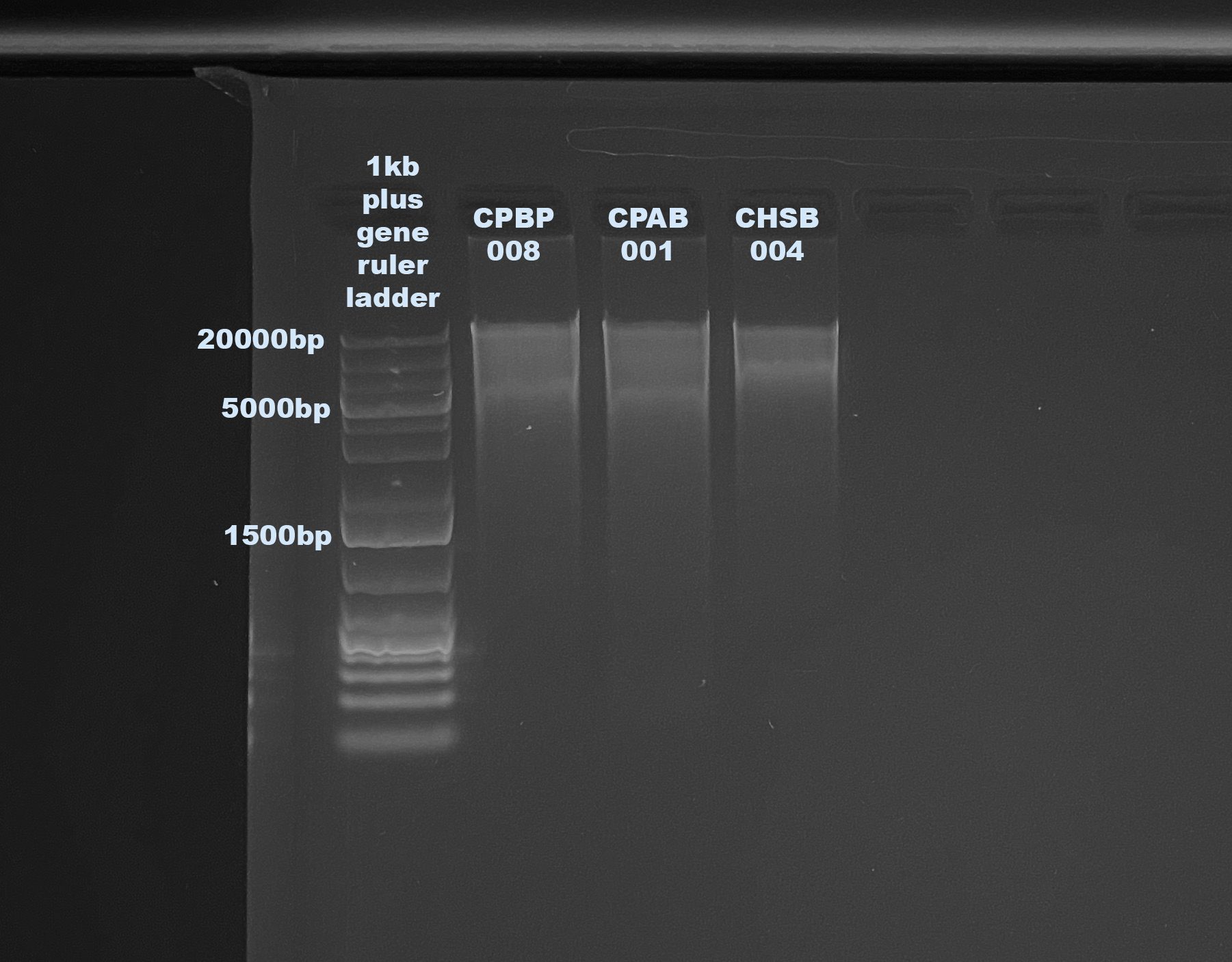
- CPBP-008
- CPAB-001
- CHSB-004
- Thawed samples on ice bucket
- Prepared forceps, foil, and razor blades
- Cleaned forceps with 10% bleach, DI water, 70% ethanol, and RNaseZap before and between each sample
- Made 1 ceramic bead tube with 800ul DNA/RNA shield for each tissuelyser sample (as per the updated kit recomendations)
- Cut a small piece of tissue for each sample then minced with the razor blade until it was flat and could come apart a little bit and placed in their respective bead homogenization tube
- CPBP-008 tissue piece and minced piece

- CPAB-001 tissue piece and minced piece

- CHSB-004 tissue piece and minced piece

- Bead tubes before tissuelyser homogenization:
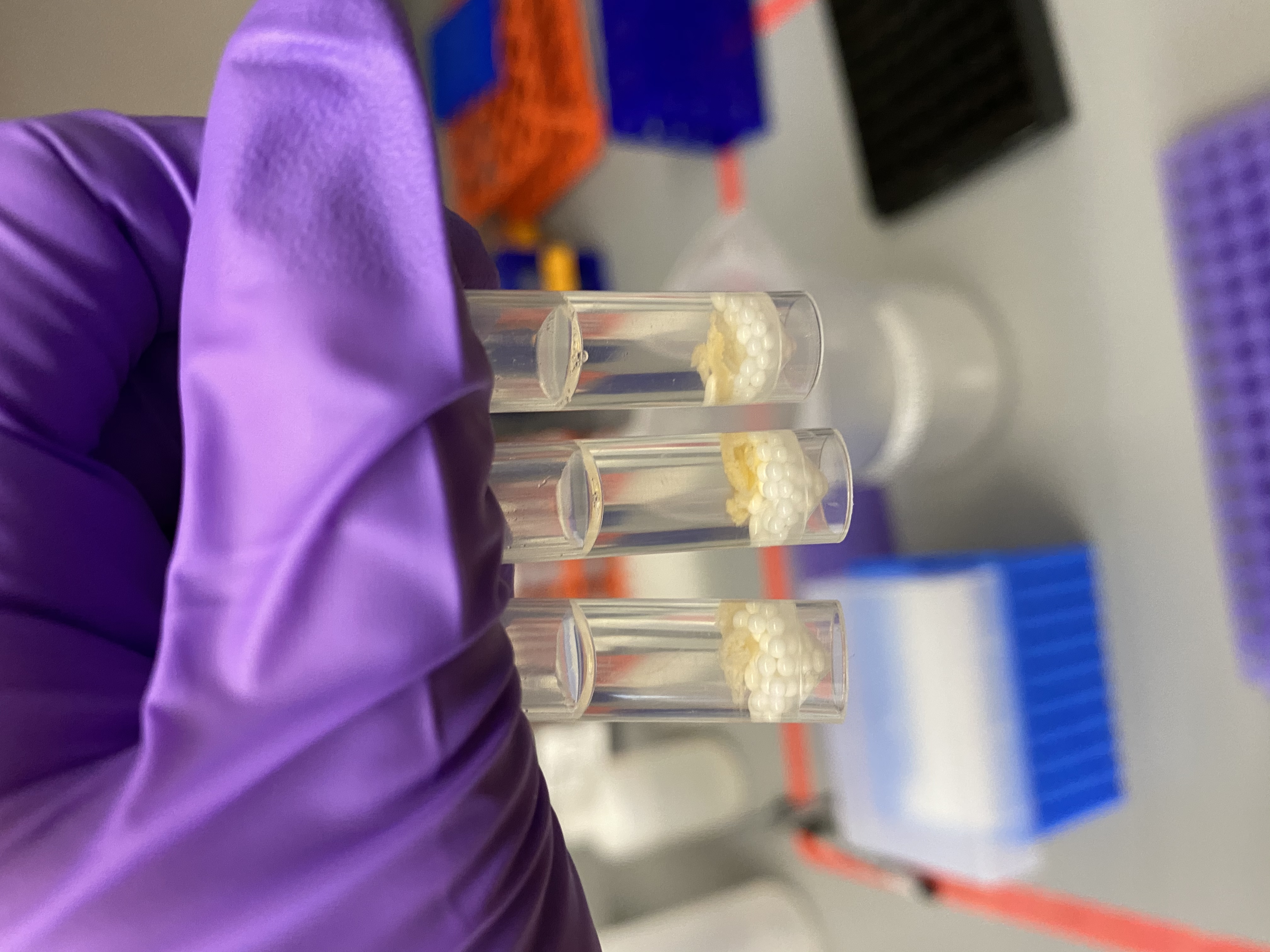
- Put bead tubes in the tissuelyser for 30 seconds at 15Hz
- Orientation of bead tubes in holders:

- After 30 seconds, changed orientation of bead tubes in holders:

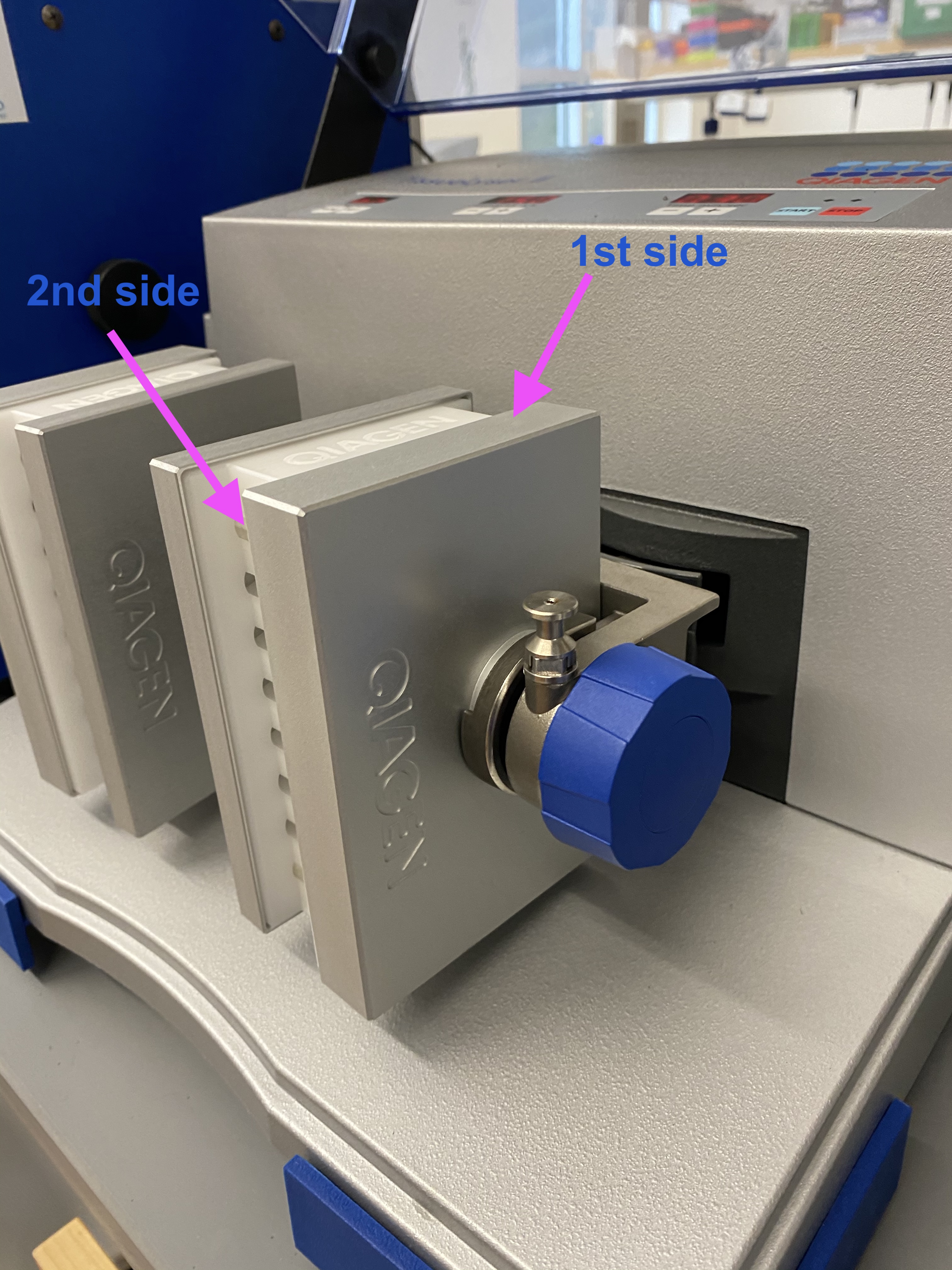
- Ran the tissuelyser again for 30 seconds at 15Hz
- Spun down the bead tubes in the minifuge to remove bubbles
- After homogenization:
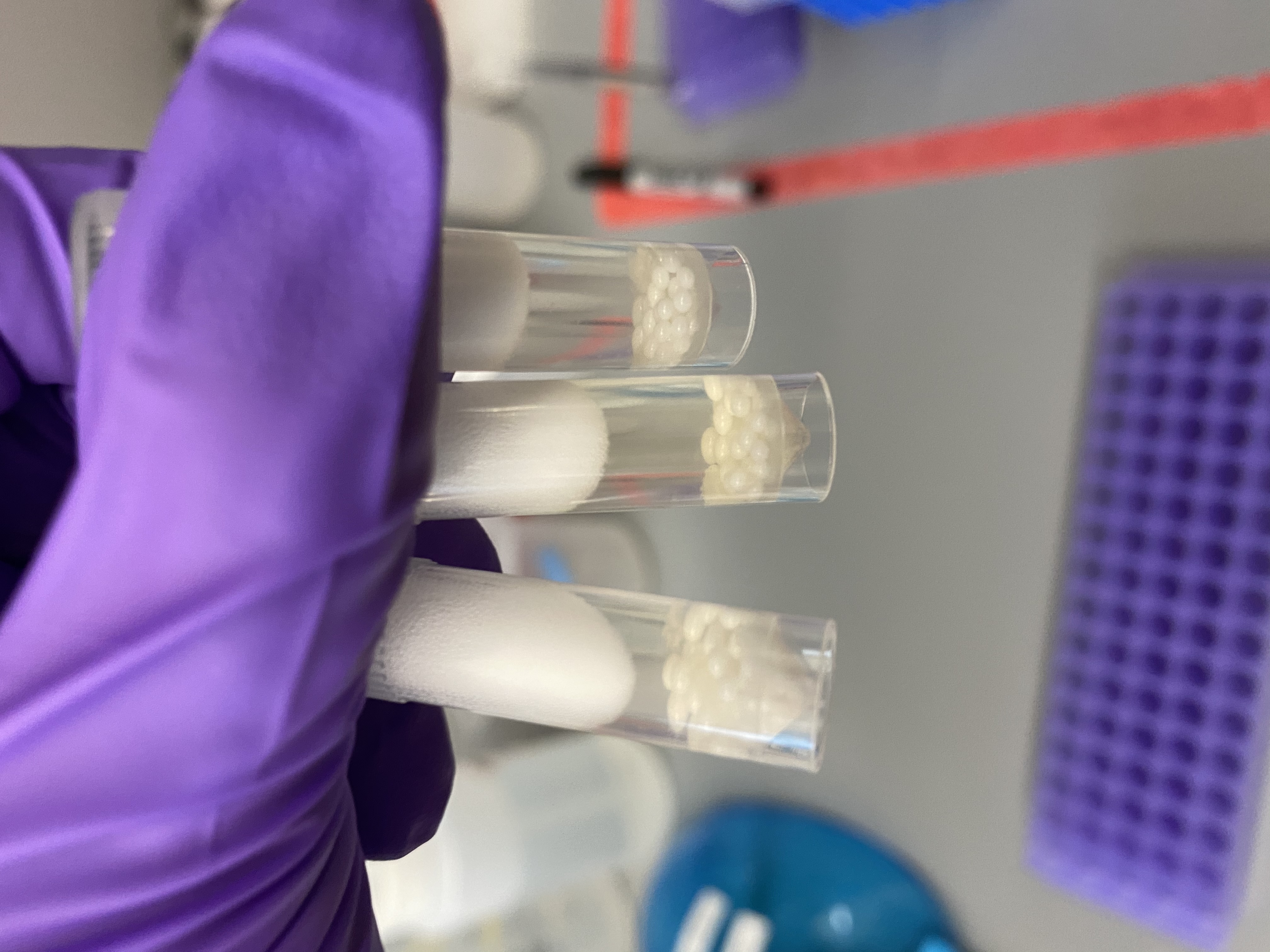
- Added 80ul proteinase K digestion buffer and 40ul proteinase K to each bead tube
- Briefly vortexed and spun down sample tubes
- Placed in the thermomixer for 10 minutes shaking at 550rpm at 22 degrees C temp (room temp)
- At 10 minutes checked on the status of the tissue:

- Saw still some tissue so decided to go to 15 minutes
- Status of tissue at 15 minutes:
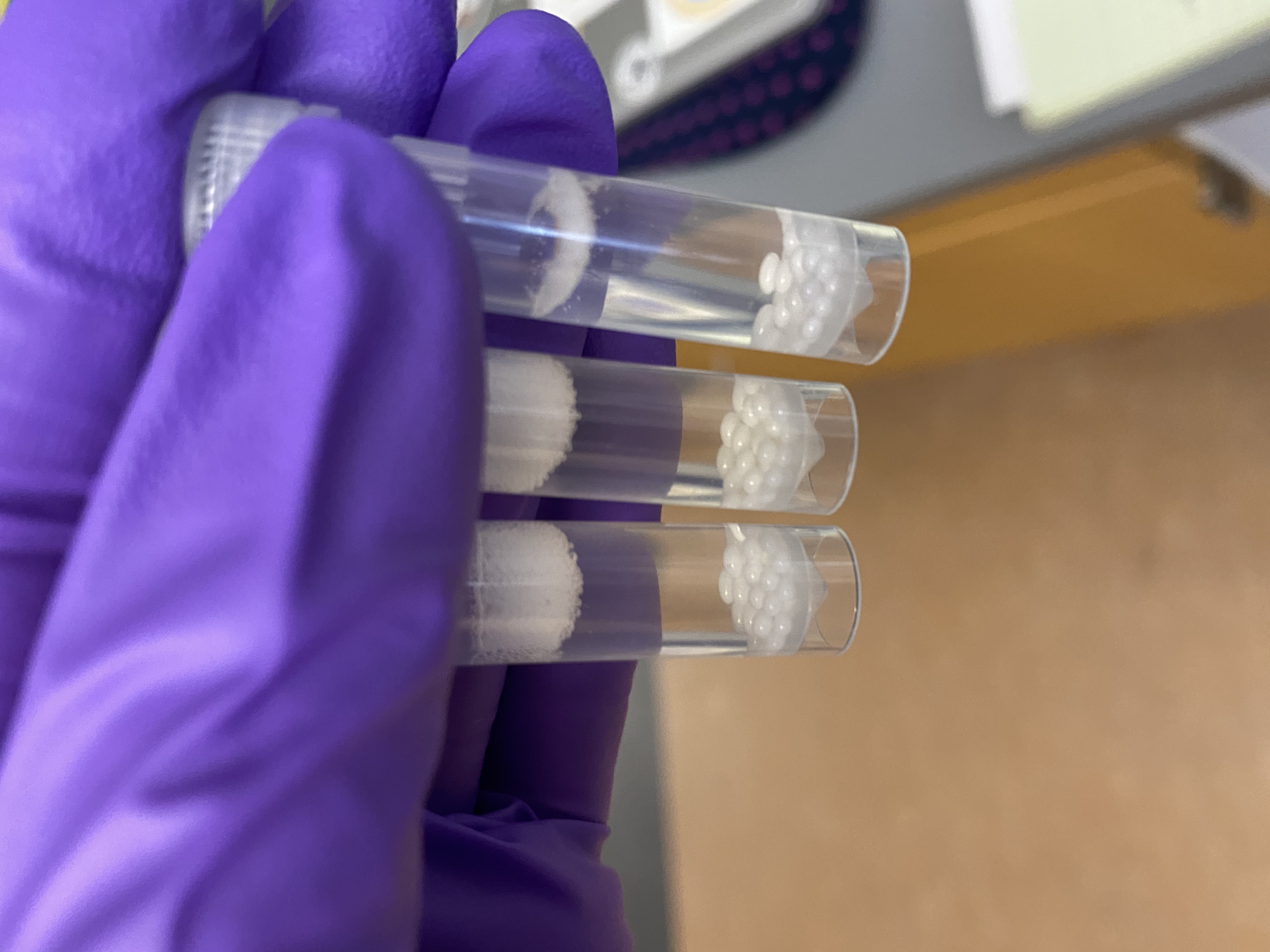
- Tissue looked pretty broken down, hard to tell if it was just in between the beads
- Removed all the supernatant (~830ul) into new 1.5mL tubes
- Centrifuged tubes for 1 minute at 16,00rcf
- Noticed pellet after centrifugation:
- Removed liquid (~830ul) into new 5mL tubes
- Added equal volume (830ul) DNA/RNA lysis buffer to the 5mL tubes
- Vortexed and spun down tubes
- Flicked and inverted tubes to mix and spun down
- Warmed 10mM Tris HCl pH 8 in the thermomixer at 70 degrees C note did not warm H2O this time
- Added 700ul of the liquid to yellow spin columns and collection tubes
- Centrifuged 16,000rcf for 30 seconds
- Saved the flow through in new 5mL tubes
- Repeated addition, centrifugation, and saving of flowthrough for the remaining amount of liquid (3 total spins)
- Added 400ul DNA/RNA prep buffer to the yellow spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 700ul DNA/RNA wash buffer to the yellow spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 400ul DNA/RNA wash buffer to the yellow spin columns
- Centrifuged 16,000rcf for 2 minutes
- Transferred spin columns to new 1.5mL tubes for each sample labeled as final DNA tubes
- Discarded flow through and collection tubes
- Added 50ul warmed 10mM Tris HCl to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Added another 50ul warmed 10mM Tris HCl to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Discarded the spin columns
- Made strip tubes with 7ul of each sample in them for QC
- Kept tubes on ice bucket then stored in -20 degree freezer
- Added equal volume (1660ul) 100% ethanol to the 5mL tubes of flowthrough
- Vortexed and spun down
- Added 700ul of the liquid to green spin columns and collection tubes
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Repeated addition, centrifugation, and saving of flowthrough for the remaining amount of liquid (5 total spins)
- Added 400ul DNA/RNA wash buffer to the green spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Created the DNase mix:
- 75ul DNA digestion buffer * 3 = 225ul
- 5ul DNase I * 3 = 15ul
- Flicked and spun down mix
- Added 80ul DNAse mix to each green spin column filter
- Incubated for 15 minutes at room temp
- Added 400ul DNA/RNA prep buffer to the green spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 700ul DNA/RNA wash buffer to the green spin columns
- Centrifuged 16,000rcf for 30 seconds
- Discarded flow through (Zymo kit waste)
- Added 400ul DNA/RNA wash buffer to the green spin columns
- Centrifuged 16,000rcf for 2 minutes
- Transferred spin columns to new 1.5mL tubes for each sample labeled as final RNA tubes
- Discarded flow through and collection tubes
- Added 50ul room temp nuclease free water to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Added another 50ul room temp nuclease free water to the spin columns gently by dripping over the filter
- Incubated at room temp for 5 minutes
- Centrifuged 16,000rcf for 30 seconds
- Discarded the spin columns
- Made strip tubes with 5ul of each sample in them for QC
- Kept tubes on ice bucket then stored in -80 degree freezer
QC
Broad Range Qubit for DNA and RNA
| Sample |
Reading 1 (ng/ul) |
Reading 2 (ng/ul) |
Average DNA (ng/ul) |
| Standard 1 |
199 RFU |
- |
- |
| Standard 2 |
21978 RFU |
- |
- |
| CPBP-008 |
28.4 |
28 |
28.2 |
| CPAB-001 |
26.6 |
26.4 |
26.5 |
| CHSB-004 |
13.3 |
13.1 |
13.2 |
| Sample |
Reading 1 (ng/ul) |
Reading 2 (ng/ul) |
Average RNA (ng/ul) |
| Standard 1 |
404 RFU |
- |
- |
| Standard 2 |
8406 RFU |
- |
- |
| CPBP-008 |
too low |
- |
- |
| CPAB-001 |
11 |
10.8 |
10.9 |
| CHSB-004 |
too low |
- |
- |
1% Agarose Gel for DNA Quality
RNA TapeStation
Written on November 12, 2020