Block 10 EecSeq Probe Synthesis, Hybridization, and Capture
Synthesis of EecSeq Probes from Block 10 cDNA Libraries and Hybridization of Probes to DNA Libraries
Normalization
Pooling of cDNA libraries, 200ng each
| Sample | μl cDNA |
|---|---|
| B10T24J1 | 6.21 |
| B10T24J2 | 6.29 |
| B10T24J3 | 4.33 |
| B10T24J4 | 5.9 |
| B10T24J5 | 8.85 |
| B10T24J6 | 6.37 |
| B10T24J7 | 6.85 |
| B10T24J8 | 8.77 |
| B10T24J9 | 2.49 |
| B10T24J10 | 2.3 |
| B10T24J11 | 3.18 |
| B10T24J12 | 3.31 |
Total Volume: 64.85μl
BR Qubit quant of pool: 40.8ng/μl
DSN Treatment
- 500ng of pool used for DSN treatment: 12.5μl in a new PCR tube
- Brought up to 13.5μl with 1μl nuclease-free H20
- 4X Hybridization buffer made
- 4.5μl of 4X hybridization buffer added to the tube
- Thermocycler DSN program
- Diluted 10X DSN Master Buffer to 2X
- Put diluted master buffer in 2nd thermocyler at 68 degree hold for 10 min to come up to temp
- Added whole volume of 2X DSN master buffer to the pooled sample in the first thermocycler, pipetted to mix inside the thermocycler
- Waited 10 minutes at 68 degrees C
- Added 2μl of DSN enzyme to pooled sample in first thermocycler, pipetted to mix with p200 pipette
- Waited 25 minutes at 68 degrees C
- Added 40μl DSN stop solution to the tube and pipetted to mix, placed on ice
Post-DSN Cleanup and PCR
- Did 1.6X clean up: 128μl KAPA Pure Beads
- Eluted in 25μl 10mM Tris HCl
- 20μl DSN treated library
- Reconstituted and diluted universal i5i7 reamp primers note, accidentally at first did not dilute properly to 20uM but did 40uM instead which is why even with adding half the volume of primers than usual, the PCR still didn’t work great, for the next PCR that was remedied. We though that the amplifications after DSN were not being efficient because of excess primer, so the volume was cut in half and made up by water in the mix
- PCR reaction for amplification
- 25μl KAPA ready mix
- 2.5μl diluted primers
- 2.5μl nuclease free water
- Placed in thermocycler 14 cycle DSN PCR program
- After: 1.6X cleanup: 80μl KAPA Pure Beads
- Eluted in 22μl 10mM Tris HCl
- Broad Range Qubit: 2.1ng/ul
D5000 TapeStation
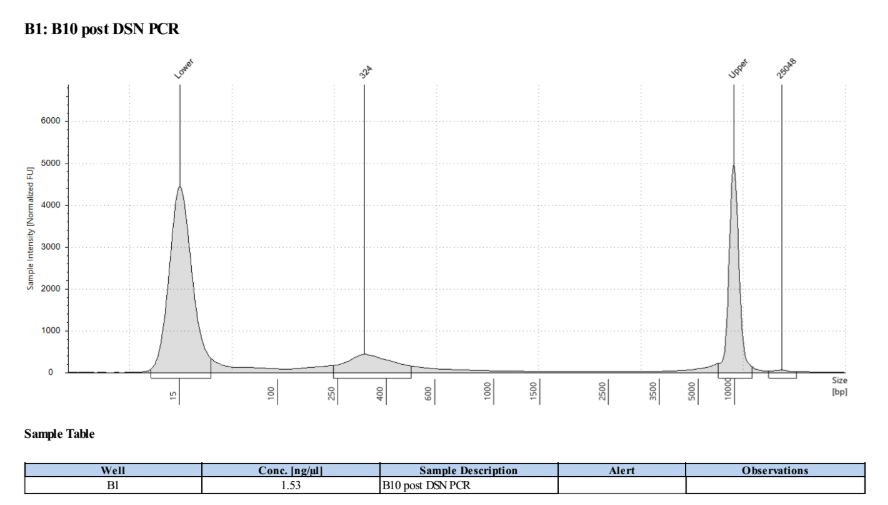
There is no primer dimer but the concentration is very low, so I decided to split it into 4 replicate tubes and doing 8 cycle PCRs on those (like the last one)
Cleanup and PCR
- Set up 4 PCR tubes with 5μl pooled-treated libs in each, saving 4μl if necessary
- PCR master mix:
- 52.5μl KAPA ready mix
- 5.25μl CORRECTLY diluted primers note: this is half volume, which is probably less than I should have used now that they are diluted properly
- 26.46μl nuclease free water
- Thermocycler 8 cycle DSN PCR program
- Pool together 4 tubes after (100μl) and 1.6X cleanup: 160μl KAPA Pure Beads
- Eluted in 40μl 10mM Tris HCl
- Broad Range Qubit: 59ng/μl
Probe Synthesis
Doing 2 separate digestions each with 1μg and saving the rest. 1μg is 16.8μl, which is more volume than the input for the digestions, but there is also water in the mix that I decided to adjust that volume to make it work.
Restriction Digest of Adapters and MBN Treatment
- Made 2 PCR tubes each with 16.8μl of pooled-treated libraries (1μg each)
- Made digest master mix:
- 8.8μl 10X cutsmart buffer
- 2.2μl SAII enzyme
- 41.14μl nuclease free water
- Added 23.65μl of mastermix to each PCR tube on ice (usually 27.7)
- Theremocycler RE digest short program 4 hours
- Made Mungbean mastermix
- 9.9μl 10X mungbean buffer
- 1.1μl mungbean nuclease enzyme
- Added 5μl of mastermix to each tube in thermocycler then did MBN program 30 min
- Did a 1.5X bead cleanup on each tube: 67.5μl KAPA Pure Beads
- eluted in 21μl 10mM Tris HCl
- Did another 1.5X bead cleanup on each tube: 31.5μl KAPA Pure Beads
- Eluted in 22μl 10mM Tris HCl and placed in fridge overnight
D5000 Tapestation the next day results look right, no left over adapters, and the size has shifted smaller with the loss of the adapters, looks good to go forward for 2 biotin labelings.
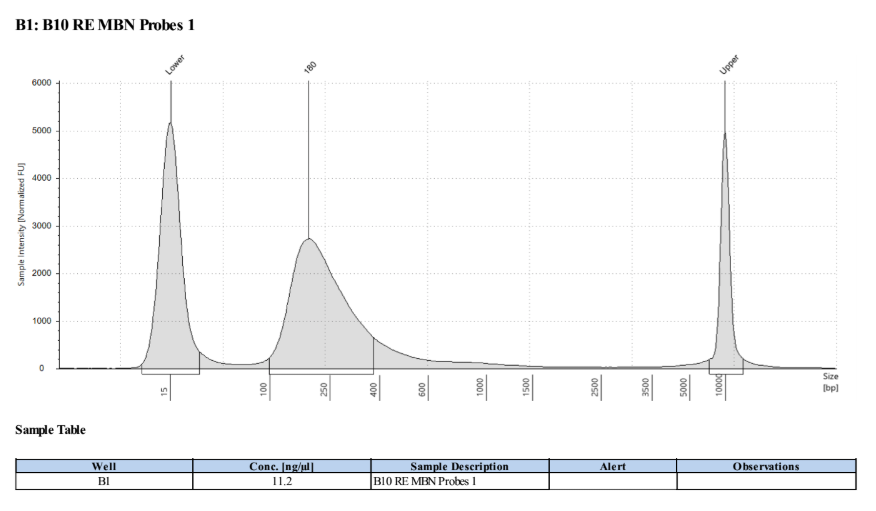
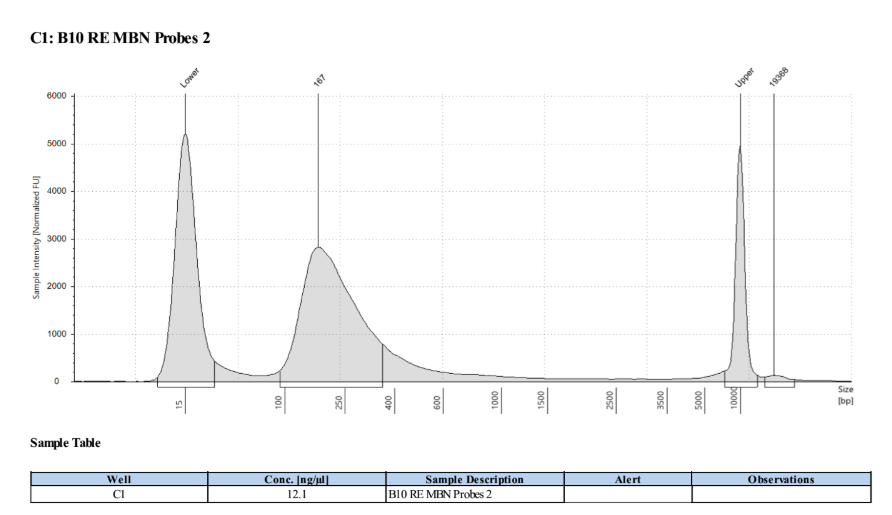
Biotin Labeling
- Made biotin mastermix:
- 22μl 5X decanucleotide reaction buffer
- 30.8μl nuclease free water
- Added 24μl of mix to 2 PCR tubes
- Added 20μl of DSN-RE-MBN treated pooled libraries, one for each tube
- Put in thermocycler DSN program, ~10 min until a 4 degree hold
- Make biotin labeling mastermix:
- 11μl biotin labeling mix
- 2.2 klenow fragment
- Added 6μl of biotin labeling master mix to each of the 4 tubes
- Shook tubes and spun down, continued program ~20 hours, done at 7:30am
- Pooled all samples together for 1.5X cleanup: 150μl KAPA pure beads
- Eluted in 50μl 10mM Tris HCl pH 8
- Qubit: 50ng/μl
D5000 Tapestation results looks normal!
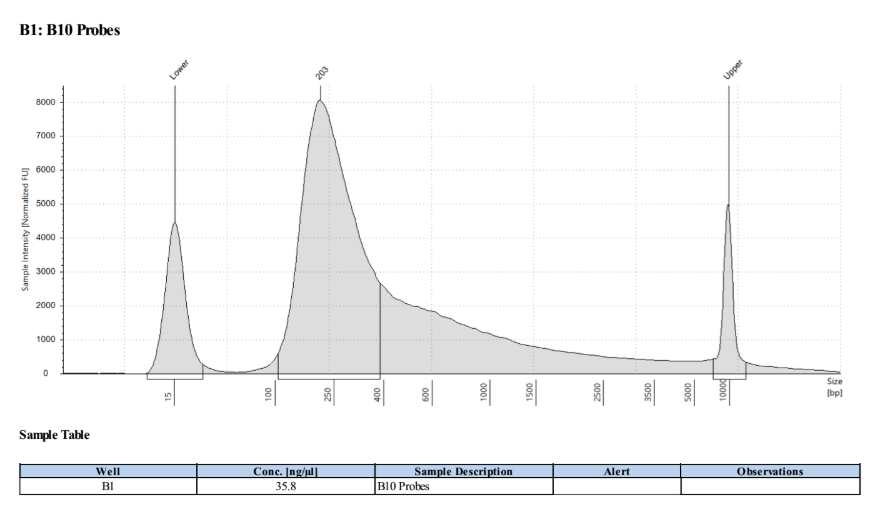
Prep and Hybridization
Pooling of DNA Libraries
- Pool 200ng of each DNA library, add 500ng of probes, then add water to equal up to 23.5μl total volume
- Probes quant is 50ng/μl, so 500ng is 10μl
- 3 captures this time
Capture 1
| Sample | vol DNA for 200ng |
|---|---|
| T0S1 | 2.99μl |
| T0S4 | 1.49μl |
| T24J1 | 1.17μl |
| T24J2 | 1.74μl |
| T24J3 | 1.21μl |
| T24J5 | 1.23μl |
| Total | 9.83μl |
Capture 2
| Sample | vol DNA for 200ng |
|---|---|
| T0S2 | 1.34μl |
| T24J4 | 1.45μl |
| T24J6 | 1.68μl |
| 24J7 | 1.38μl |
| T24J8 | 1.33μl |
| T24J17 | 1.41μl |
| Total | 8.59μl |
Capture 3
| Sample | vol DNA for 200ng |
|---|---|
| T0S3 | 1.23μl |
| T24J9 | 1.18μl |
| T24J10 | 1.23μl |
| T24J11 | 1.21μl |
| T24J12 | 1.42μl |
| T24J13 | 1.2μl |
| Total | 7.47μl |
- 10μl of block 10 probes were added to each capture pool
- The volume of nuclease free water needed to add up to 23.5μl total volume in each capture pool was calculated then added:
| Sample | vol pool w/probes | vol H20 |
|---|---|---|
| Cap 1 | 19.83μl | 3.67μl |
| Cap 2 | 18.59μl | 4.91μl |
| Cap 3 | 17.47μl | 6.03μl |
Hybridization
- Made hybridization master mix (volumes for 1 hybridization multiplied by 4.3):
- 39.6μl 20X SSC
- 1.32μl 500mM EDTA
- 1.32μl 10% SDS
- 5.28μl 50X Denhardt’s solution
- 1.65μl human COT-1 DNA
- 1.32μl blocking oligo 1
- 1.32μl blocking oligo 2
- 1.32μl blocking oligo 3
-
1.32μl blocking oligo 4
- Added 16.5μl of hybridization mix to each capture pool and pipetted to mix note the mix had a white precipitate that mostly vortexed evenly through the solution
- Put in the thermocycler hybridization program 95C for 10 minutes then moved to the already warmed incubator genie 65C rocking 10 speed for 48 hours, I tried to parafilm the lids to protect from evaporation. Samples were taken out and vortexed and spun down once ~24 hours in
Washes and Solutions for Capture
TEN
- 10mM Trish HCl pH 7.5, 1mM EDTA, 1M NaCl
- Need 2800μl
- 5.6μl of 500mM EDTA
- 560μl of 5M NaCl
- 2234.4μl of 10mM Tris HCl pH 7.5
Solution 1
- 1X SSC, 0.1% SDS
- Warmed to 65C in thermomixer in a 1.5mL tube
- Need 1400μl (used twice)
- 70μl 20X SSC
- 14μl 10% SDS
- 1316μl nuclease free H20
Solution 2
- 0.5X SSC, 0.1% SDS
- Need 700μl
- 17.5μl 20X SSC
- 7μl 10% SDS
- 675.5μl nuclease free H2O
Solution 3
- 0.1X SSC, 0.1% SDS
- Need 700μl
- 3.5μl 20X SSC
- 7μl 10% SDS
- 689.5μl nuclease free H2O
Prepare Beads
- Resuspended Dynabeads M-280 beads first with p200
- Made 3 PCR tubes with 10μl of resuspended beads in each
- Added 200μl of prepared TEN solution to each tube and pipetted to mix
- Placed tubes on magnet plate and removed supernatant when clear
- Removed from magnet and resuspended beads in 200μl TEN solution
- Repeated wash twice for a total of 3 washes
- Resuspended beads in 200μl TEN solution
Capture
- After the ~48 hour hybridization incubation, took the hybridized pools out of the incubator, spun them down, and added the 4 hybridization mixtures to each of the 4 washed and prepared Dynabead PCR tubes note: volume in hybridization tubes was less than 40, potentially there was evaporation
- Pipetted to mix and incubated on the shaker at room temp for 30 minutes
- Placed solution 1 in thermomixer to get to 65°C
- Turned on thermocycler and set it on the 65°C hold program
-
Made PCR strip tubes to save every wash (20 total tubes)
- Placed tubes on magnet plate after incubation
- Removed supernatant when clear and saved as “start”
- Resuspended beads in 200μl 65°C solution 1
-
Placed tubes in thermocyler set at 65°C for 15 minutes
- Placed tubes on magnet plate after incubation
- Removed supernatant when clear and saved as “wash 1”
- Resuspended beads in 200μl 65°C solution 1
-
Placed tubes in thermocyler set at 65°C for 10 minutes
- Placed tubes on magnet plate after incubation
- Removed supernatant when clear and saved as “wash 2”
- Resuspended beads in 200μl room temp solution 2
-
Placed tubes in thermocyler set at 65°C for 10 minutes
- Placed tubes on magnet plate after incubation
- Removed supernatant when clear and saved as “wash 3”
- Resuspended beads in 200μl room temp solution 3
-
Placed tubes in thermocyler set at 65°C for 10 minutes
- Turned on second thermocycler and set it on the 80°C hold program
-
Placed 200μl of nuclease free H20 in the 80°C thermocycler
- Placed tubes on magnet plate after incubation
- Removed supernatant when clear and saved as “wash 4”
- Resuspended beads in 22μl 80°C nuclease free water
-
Placed tubes in thermocyler set at 80°C for 10 minutes
- Made PCR master mix for amplification:
- 12.5μl KAPA ready mix * 3.3 = 52.5μl
- 2.5μl KAPA universal primer mix * 4.5 = 10.5μl
-
Aliquoted 15μl of PCR master mix into each of 4 PCR tubes labeled for each capture
- Placed Dynabead tubes on magnet plate after incubation
- SAVED supernatant when clear as captured DNA into new PCR tubes
- 10μl of captured DNA was transferred to their corresponding PCR tubes containing the amplification master mix and were vortexed and spun down
- Tubes for amplification were placed in the thermocyler for the post-capture 12-cycle PCR program
- The other tubes (all washes and the remaining ~10μl of captured DNA) were placed in the -20 freezer
- After the PCR, a 1X bead cleanup was performed:
- Added 25μl of KAPA pure beads to each sample
- Performed normal bead cleanup
- Resuspended and retained captured DNA in 25μl 10mM Tris HCl
High Sensitivity Qubit:
| standard 1 | standard 2 | Capture 1 post-PCR | Capture 2 post-PCR | Capture 3 post-PCR |
|---|---|---|---|---|
| 50.77 RFU | 26616 RFU | 15.7ng/μl | 13.9ng/μl | 13.75ng/μl |
D5000 TapeStation to check that the right stuff was in the sample:
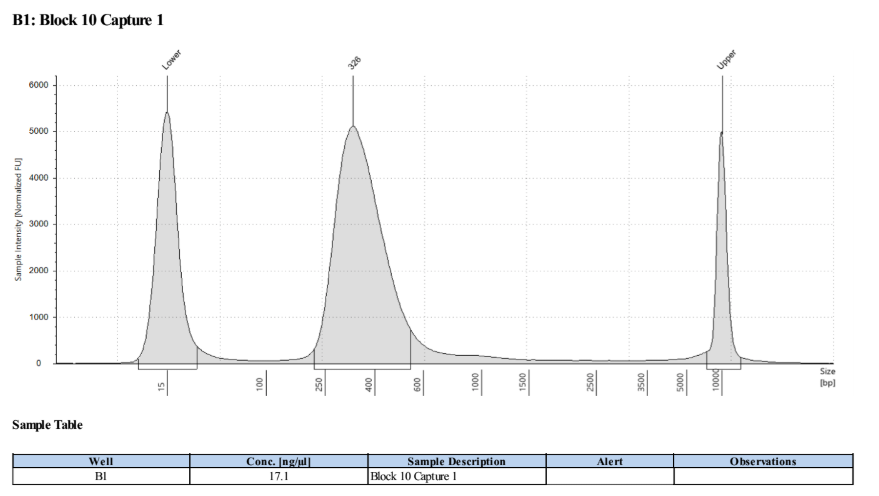
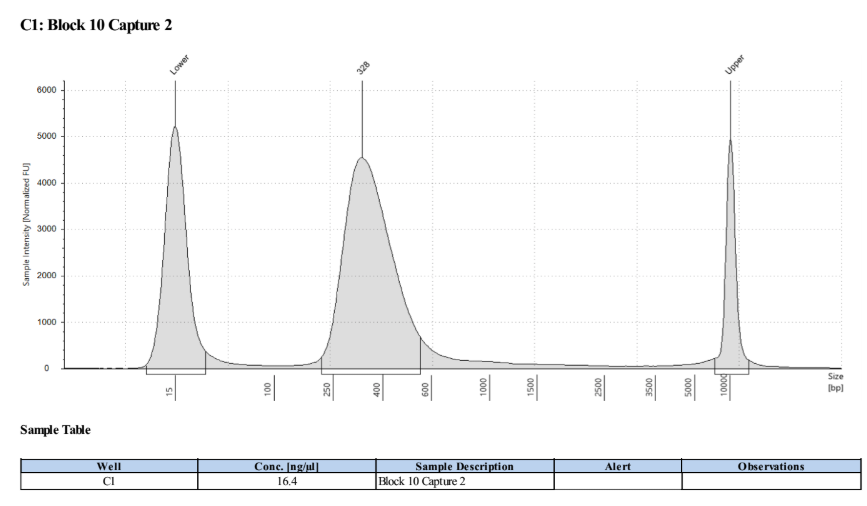
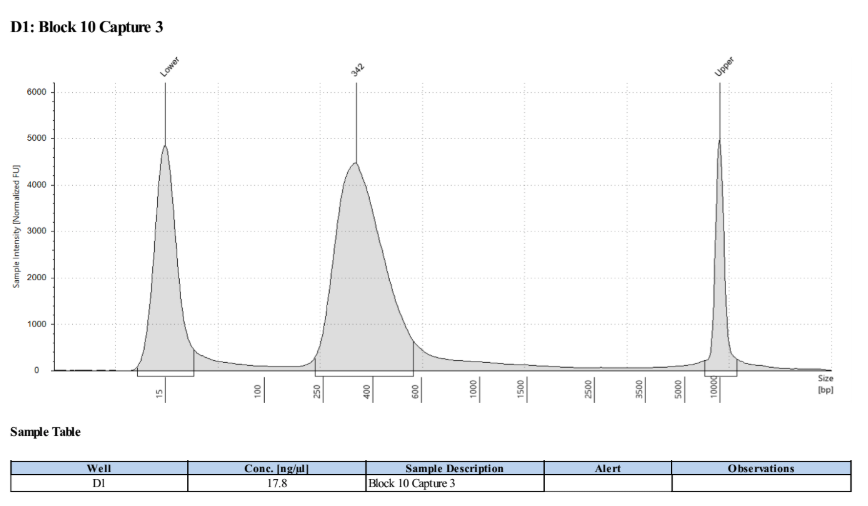
full results