3rd Try Porites astrodies RNA Extraction with Purelink Kit
Trying Extraction Again With RNA Purelink Kit to Get RNA from 1 Porites asteroides Samples
In this Extraction I used: Purelink RNA mini Kit, Trizol Reagent, 1-Bromo-3-cholropropane, and a protocol from Federica. I modified the protocol by grinding a large chunk on LN2, increasing the initial volume of trizol reagent to 1100ul and homogenizing with the tissuelyser for 4 minutes at 25Hz, switching tube positions at 2 minutes.
Goal: Extract high quality RNA out of one sample to get this protocol nailed down
Result: Again, no RNA extracted :(
Major Take Aways: Now I really don’t know what is the problem, maybe I have been using too much sample this whole time. That is my only guess
Note: Protocol is done in the hood as Trizol and 1-Bromo-3-cholropropane are toxic and corrosive. PPE of lab coat, mask, eye glasses, and gloves were worn at all times. Solid and liquid wastes are hazardous.
Sample Prep
- Sample: R5 A2 201907 Molec (used all of this in the process, it was a small chip left)
- Sterilized scrapper with 10% bleach, DI water, then 70% EtOH, and RNaseZap
- Chilled mortar and pestle in -80
- Put whole chunk of sample in chilled mortar

- Poured in LN2 and ground until it was small pieces

- Scrapped pieces into a bead tube with 800ul of trizol reagent
- There was a lot of ground tissue so I added another 300ul of trizol reagent because I wasn’t sure I would be able to get out 550ul later. Total trizol volume was 1100ul
- Put the bead tube in the tissuelyser 2 minutes at 25Hz
- Switched orientation in the tissuelyser so the tube were on the outside of the holders
- Ran the tissuelyser again for 2 minutes at 25Hz
- After tissuelyser homogenization:
Extraction
- Removed 550ul from the bead tube and put into a new 1.5mL tube
- There was still more liquid in there, so I made another 1.5ml tube with 300ul of homogenized liquid. The tubes were now labeled A and B
- Increased the volume in each 1.5mL tube to 1mL with trizol reagent: 450ul for A and 700ul for B
- Vortexed tubes briefly and spun down
- Incubated tubes at room temp for 5 minutes
- Added 100ul of 1-Bromo-3-cholropropane and vortexed tubes for 15 seconds each
- The tubes became cloudy after vortexing
- Incubated tubes at room temp for 3 minutes
- Centrifuged tubes for 15 minutes at 12,000rcf at room temp
- Prepared DNase treatment on ice:
- 16ul 10X reaction buffer
- 20ul DNAse
- 124ul RNase-free water
- After centrifugation tubes had phase separated:
- A:
- B:

- There was also a visible pellet in each tube, probably skeleton?
- A:
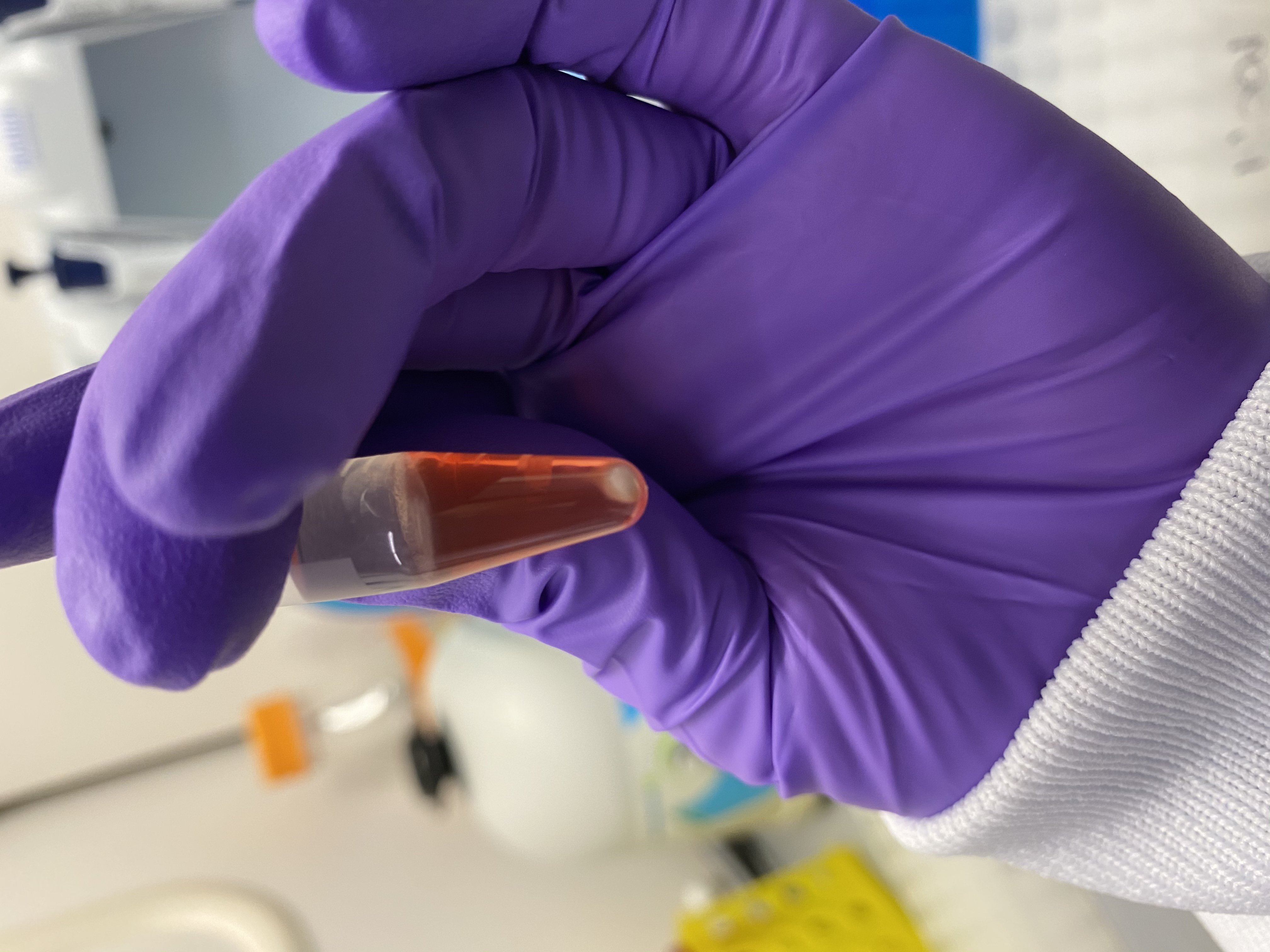
- B:

- Was able to remove ~550ul of the top clearish layer into new 1.5mL tubes (tough to remove without getting other layers)
- Added 550ul 70% ethanol to each new tube and vortexed (note, no precipitates formed)
- Added 600ul to spin columns for each sample
- Centrifuged 25 seconds at 12,000 rcf (note, I used a different centrifuge this time that took until 20 seconds had passed to get to 12,00rcf)
- Discarded flow through in trizol waste
- Added the remaining sample to their spin columns
- Centrifuged 25 seconds at 12,000 rcf
- Discarded flow through in trizol waste
- Added 350ul of wash buffer 1
- Centrifuged 25 seconds at 12,000 rcf
- Discarded flow through in trizol waste and changed collection tubes
- Added 80ul DNase mixture to each spin column and incubated at room temp for 12 minutes
- After incubation, added 350ul wash buffer 1
- Centrifuged 25 seconds at 12,000 rcf
- Discarded flow through in trizol waste and changed collection tubes
- Added 500ul wash buffer 2
- Centrifuged 25 seconds at 12,000 rcf
- Discarded flow through in trizol waste
- Again added 500ul wash buffer 2
- Centrifuged 25 seconds at 12,000 rcf
- Discarded flow through in trizol waste
- Centrifuged columns dry for 1 minute at 12,000rcf
- Transferred columns to recovery 1.5mL tubes
- Added 100ul RNase-free water to each column
- Incubated at room temp ~1minute
- Centrifuged for 2 minutes at 16,000rcf
- Aliquoited 5ul for QC and froze the big tubes in the -80
Qubit – HS Assay this time
- Followed qubit protocol
| Standard 1 | Standard 2 | Sample | RNA :( |
|---|---|---|---|
| 48 RFU | 905 RFU | R5 A | too low |
| - | - | R5 B | too low |
- Did not do a tapestation
Written on November 25, 2020