mtORF Amplification of HMW P. acuta
Amplifying mtORF region from HMW Pocillopora Extraction and Sequencing Prep
Goal: Use extracted HMW DNA to amplify mtORF region in coral to confirm species
Result: Good yields from amplification
Major takeaways: Even though I had to dilute a lot for 10ng/ul, there were no issues
Sample Info Used coral P2185 from HoloInt Project, tube 3 from this HMW extraction
mtORF Amplification, Cleanup and Sequencing Prep
Followed the mtORF Amplification Protocol exactly. Briefly:
- Diluted samples to 10ng/ul in 10ul:
- 2ul of DNA and 38.75ul TE buffer
- Master mix for 1 triplicate reaction:
- 50ul phusion amplification mix
- 1.3ul of FatP6 primer (10uM)
- 1.3ul of RORF primer (10uM)
- 44ul ultra pure water
- Aliquoted 97ul into 1 tube
- Added 3ul of diluted DNA into the 97ul tube
- Vortexed and spun down
- Split up 100ul tubes into 3 33ul tubes
- Spun down tubes
- Placed in the thermocyler FATP RORF program
- Afterwards, combined triplicate samples and added 100ul of KAPA Pure Beads for a 1X clean up
- Performed normal cleanup
- Eluted in 50ul of ultra pure water
- Qubit of amplified DNA (protocol):
| Sample | DNA Standard 1 (RFU) | DNA Standard 2 (RFU) | DNA 1 (ng/µl) | DNA 2 (ng/µl) | Average DNA (ng/ul) |
|---|---|---|---|---|---|
| P2185 | 189 | 20669 | 61 | 61 | 61 |
- 1% gel run at 100V for 60 minutes, band size is expected to be 1000bp:
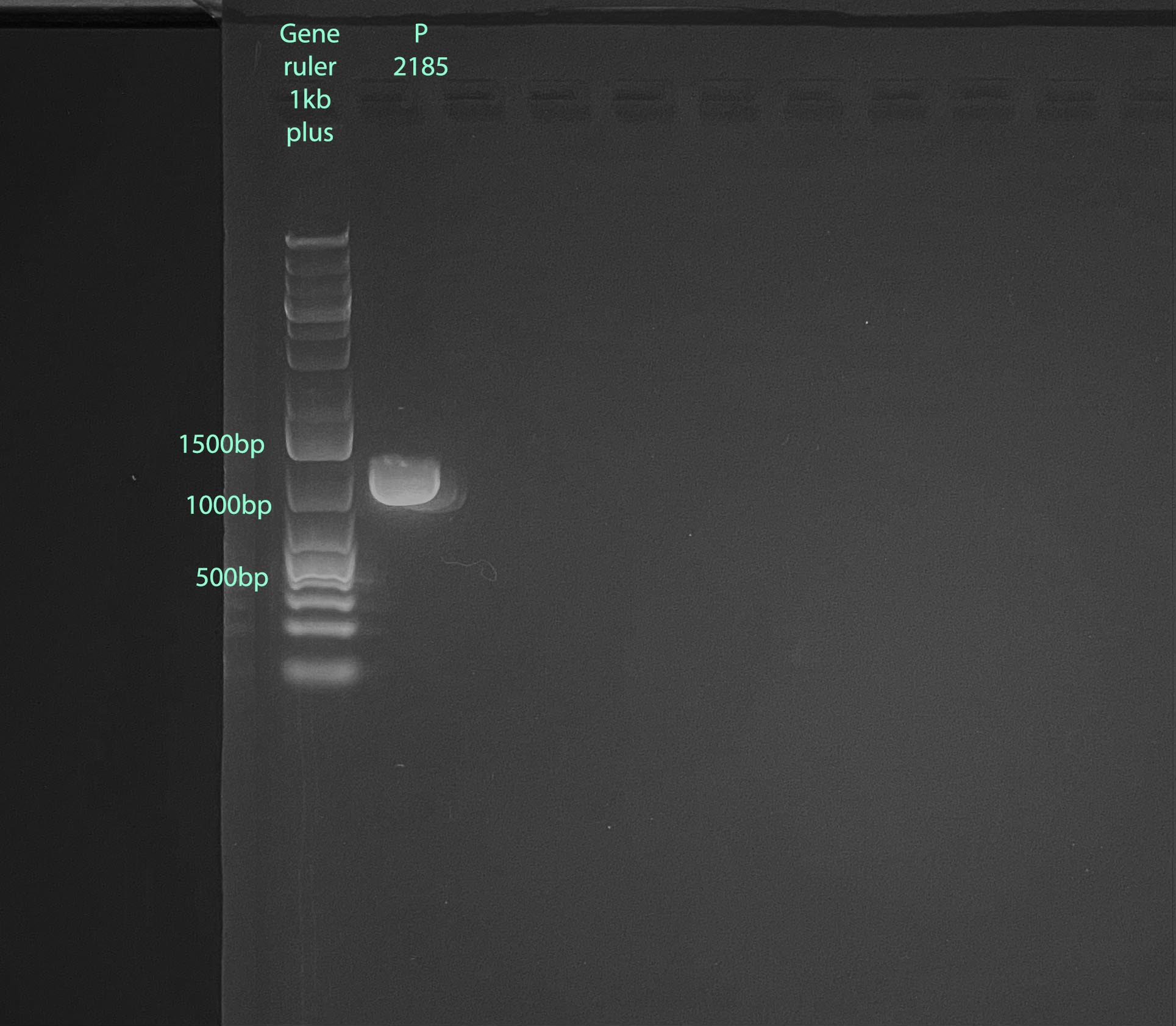
- 2ul of 3.2mM FATP6.1 primer is needed, 5ul was made
- 1.6ul of 10mM primer
- 3.4ul of ultra pure water
- 1:10 dilution of amplified DNA
- 2ul of DNA
- 18ul of ultra pure water
- New concentration should be:
- 6.1ng/ul
- Amount of DNA and water for 10ul containing 25ng of DNA:
- 4.1ul of diluted DNA and 5.9ul of ultra pure water
- Added 2ul of the 3.2mM FATP6.1 primer to each tube
- Spreadsheet for GSC:
| Sample IDa | Well (GSC use only) | Template Typeb | A. Template Size (bases) | B. Template Stock Conc. (ng/µl) | C. PCR template: ng needed = ((A ÷ 100) x 1.25) x 2 | D. PCR template: Volume = (C ÷ B) µl | F. Volume PCR-H20 needed (10 minus D or E) µl | G. Volume primer needed 1 µl per reaction |
|---|---|---|---|---|---|---|---|---|
| HAQ3 | PCR | 1000 | 6.1 | 25 | 4.1 | 5.9 | 2 |
- Brought up to GSC 20210222
Written on February 22, 2021