Testing Sonication for MBD
Sonication Tests with Q800R3 Sonicator
Using two samples to test optimal time and intensity for shearing coral DNA for MBD library prep
Samples:
Acropora sperm # 221 (High molecular weight DNA)
Montipora soft homogenization # 1431 (smeared DNA)
Using 1µg of DNA in 50µl of ultra pure water in these tubes
| Sample | Vol DNA to 1µg | Vol H20 to 50µl | Tube # |
|---|---|---|---|
| 221 | 7.69µl | 42.31µl | 9 |
| 1431 | 5.21µl | 44.79µl | 10 |
| 221 | 7.69µl | 42.31µl | 11 |
| 1431 | 5.21µl | 44.79µl | 12 |
| 221 | 7.69µl | 42.31µl | 13 |
| 1431 | 5.21µl | 44.79µl | 14 |
- Used QSonica protocol to set up the sonicator
- Set the amplitude to 25%
- Set the pulse to be 15 seconds on and 15 seconds off
- Used 3 different time setting to test what would give us shearing to about 500 basepairs (1 minute setting, 2.5 minute setting, and 1.5 minute settings)
- A cycle is defined as 15 seconds on and 15 seconds off (so 30 seconds total)
- 1 minute and 2.5 minute settings were done first, visualized, and then we decided to try in the middle with a 1.5 minute setting
| Sample | Tube # | Time Setting | # of Cycles |
|---|---|---|---|
| 221 | 9 | 1 min | 4 cycles |
| 1431 | 10 | 1 min | 4 cycles |
| 221 | 11 | 2.5 min | 10 cycles |
| 1431 | 12 | 2.5 min | 10 cycles |
| 221 | 13 | 1.5 min | 6 cycles |
| 1431 | 14 | 1.5 min | 6 cycles |
- Visualization with D5000 tapes for the TapeStation 4200 (protocol)
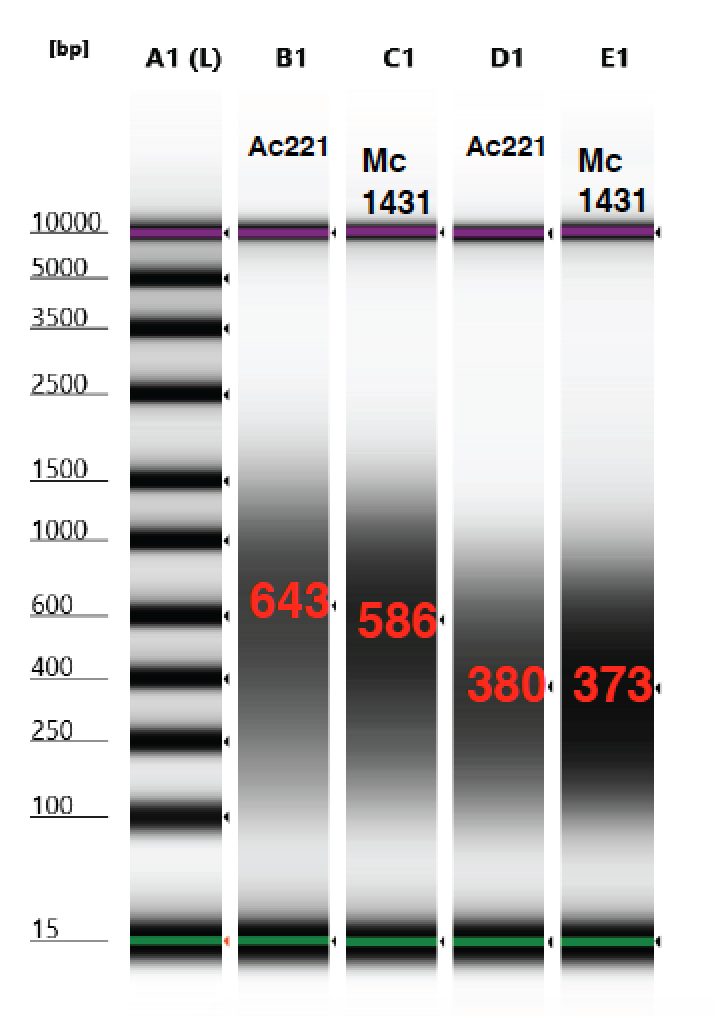
1 minute setting and 2.5 minute setting results
1.5 minute setting results
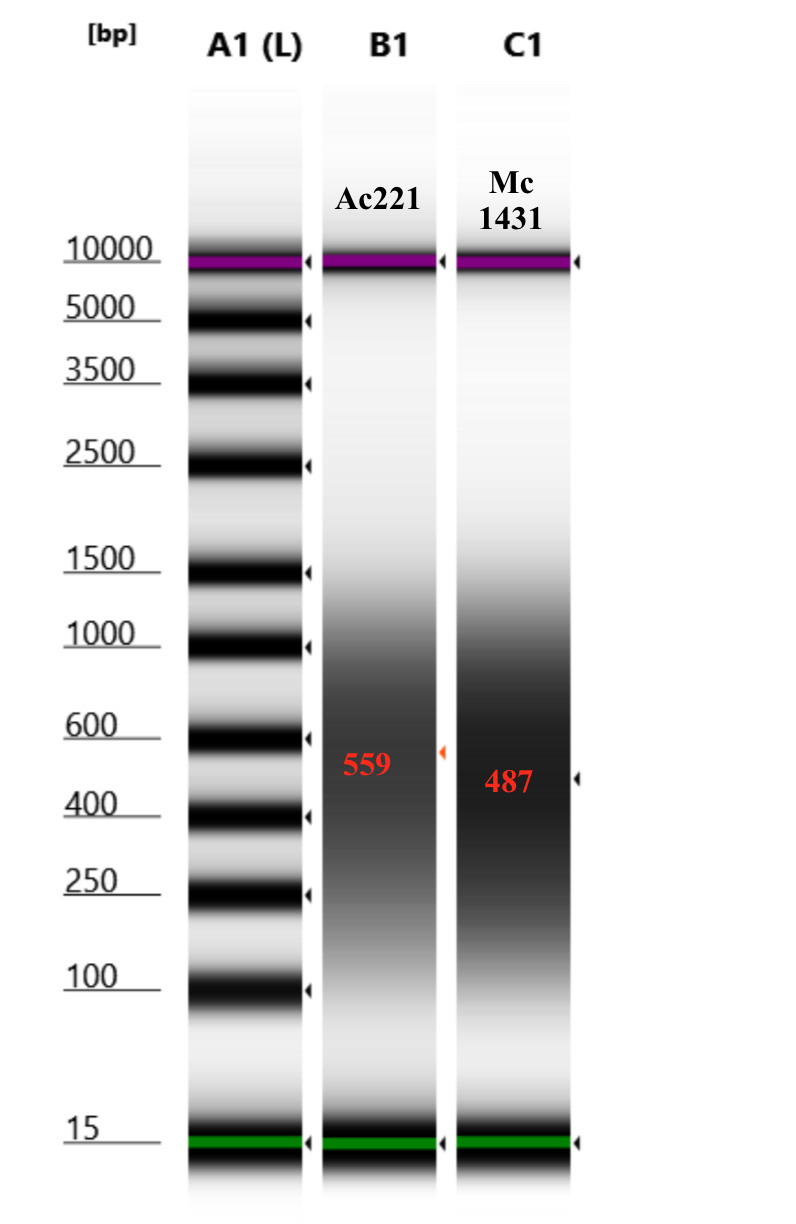
1.5 minute setting seems to be the best option for getting around 500 bp, for both starting HMW and smeared, even though there are differences
Written on July 30, 2019