Troubleshoot and Redo Some Samples for Methylation Comparison With Pico Methyl Seq Library Prep Kit
Additional amplifications of already made libraries and two re-tries of the full library prep with samples from the previous post. Using the Zymo Pico Methyl Seq Kit for the purpose of comparing to RRBS preparations
Samples for methylation comparison are from the Holobiont Integration Project, and were extracted by Emma Strand or myself, see her notebook posts for the extraction specifications: 20190805 and 20180823, 20190718 and 20190903.
Re-Amplification on 10-2-19
Original amplifications of library preps could have been low for all samples because we added too much primer (see primer dimer present in all sample traces in previous post). All samples except WGBS 1471 were amplified again and then bead cleaned to be sure to remove any primer dimer. Samples were also bead cleaned before amplification to ensure remaining primer was not inhibiting anything.
1X bead clean
- 11µl of KAPA pure beads to each sample (1X)
- Performed normal clean up with fresh 80% EtOH
- Resuspended and eluted in 12µl of DNA elution buffer from the Pico Methyl-Seq kit
Re-amp
- Some already made libraries needed more amplification than others, they were split into an extra 4 cycle PCR or extra 2 cycle PCR
- The same index primers were used but only 0.5µl of each were added this time
- Set up new strip tubes with:
| Sample | vol library needed | vol lib amp master mix | vol i5 index | vol i7 index | # of cycles |
|---|---|---|---|---|---|
| 1041 WGBS | 12µl | 13µl | .5µl 1 | .5µl 1 | 4 |
| 1101 WGBS | 12µl | 13µl | .5µl 10 | .5µl 11 | 4 |
| 1101 Captured MBD | 12µl | 13µl | .5µl 16 | .5µl 16 | 4 |
| 1548 Captured MBD | 12µl | 13µl | .5µl 17 | .5µl 17 | 4 |
| 1628 Captured MBD | 12µl | 13µl | .5µl 18 | .5µl 18 | 4 |
| 1637 WGBS | 12µl | 13µl | .5µl 3 | .5µl 3 | 2 |
| 1548 WGBS | 12µl | 13µl | .5µl 11 | .5µl 10 | 2 |
| 1628 WGBS | 12µl | 13µl | .5µl 12 | .5µl 12 | 2 |
| 1041 Captured MBD | 12µl | 13µl | .5µl 7 | .5µl 7 | 2 |
| 1471 Captured MBD | 12µl | 13µl | .5µl 8 | .5µl 8 | 2 |
| 1637 Captured MBD | 12µl | 13µl | .5µl 9 | .5µl 9 | 2 |
- PCR programs were copied from the last amplification program in the Pico Methyl-Seq protocol and # of cycles was adjusted
After-amp 1X bead clean
- 26µl of KAPA pure beads to each sample (1X)
- Performed normal clean up with fresh 80% EtOH
- Resuspended and eluted in 14µl of DNA elution buffer from the Pico Methyl-Seq kit
- Ran D5000 tapestation
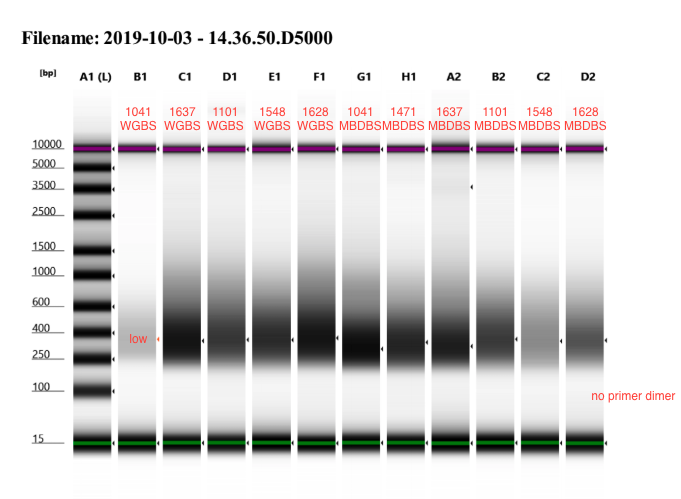
- 1041 WGBS looks like it didn’t amplify well again
Re-Library prep 1041 and 1471 WGBS
- Re-doing the library prep for 1041 which did not amplify well and 1471 which did not work at all
- Followed exact same steps as in this library prep except did a 1X bead clean up after final amplification instead of doing the DCC
- Ran D5000 Tapestation
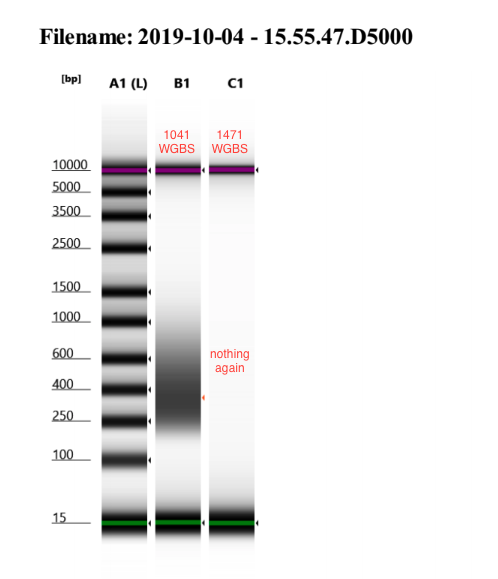
- Once again 1471 WGBS did not work
One more try of 1471 WGBS and re-do of 1101 and 1548 WGBS with correct paired indexes this time
- Re-diluteded i5 #2 and i7 #2 indexes to 10uM to see if the primers were the issue
- Followed exact same steps as in this library prep except did a 1X bead clean up after final amplification instead of doing the DCC
- 1101 WGBS now has paired #10 i5i7 indexes and 1548 now has paired #11 i5i7 indexes
- Ran D5000 Tapestation
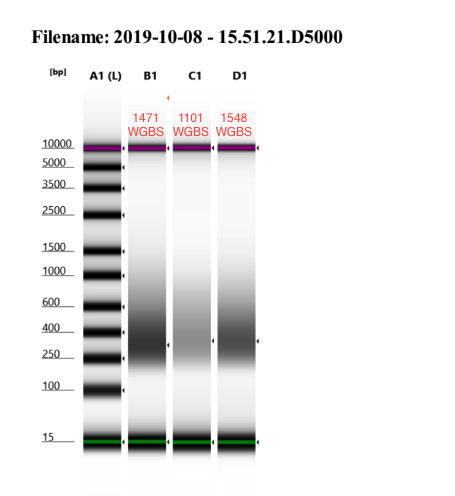
Library concentrations for all completely libraries for the methylation methods comparison project. All libraries are in 13µl DNA elution buffer from the Zymo kit
| Sample | Library Concentration |
|---|---|
| 1041 WGBS | 10.5ng/ul |
| 1471 WGBS | 11.9ng/ul |
| 1637 WGBS | 18.7ng/ul |
| 1101 WGBS | 18.1ng/ul |
| 1548 WGBS | 15.6ng/ul |
| 1628 WGBS | 13.7ng/ul |
| 1041 MBDBS | 5.95ng/ul |
| 1471 MBDBS | 10.2ng/ul |
| 1637 MBDBS | 18.4ng/ul |
| 1101 MBDBS | 12.3ng/ul |
| 1548 MBDBS | 5.61ng/ul |
| 1628 MBDBS | 9.39ng/ul |