Using the Qiagen Genomic Tip for HMW DNA Extraction of Porites asteroides
High Molecular Weight DNA Extraction of Porites asteroides with the Qiagen Genomic Tip for Intended Genome Sequencing
In this protocol I use the QIAGEN Genomic-tip 100/G, the QIAGEN Genomic DNA Buffer Set, QIAGEN RNase A (100mg/mL concentration), and QIAGEN Proteinase K
This is from the successful attempt at extracting, the day before there was an unsuccessful attempt
Sample Chiseling
Set up
- Prepared buffer with 9.5mL of Buffer G2 and 19ul of RNase A
- Set incubator genie to 50 degrees C
- Cleaned chisel, forceps, scraper/scooper and bench with 10% bleach, DI water, then 70% EtOH
- Wrapped those in foil and placed in -80
- Also placed mortar and pestle in -80
- Had styrofoam cooler of dry ice saved and LN2 in the Thermoflask dewar
- Brought over scale
- Set up dry ice box with plastic box rack over the ice to have a hard surface for chiseling, then covered that in foil
Chiseling
- Amy Zyck helped by holding the nubbin (literally unable to chisel without someone holding it)
- Took out nubbin: TA HOT 1 and unwrapped from foil (sample was flash frozen and kept at -80)
- Chiseled with large chisel and hammer until a few chunks came off
- Put mortar on the scale and tarred
- Used forceps to put chunks into mortar: 0.11g
- Decided to do a few more chunks (kit recommends 100mg, but hard to say how much skeleton I’m getting and how much will get scrapped out of the mortar)
- While chiseling again added LN2 to mortar to keep original chunks chilled
- Final weight 0.18g
- Poured LN2 into mortar and let boil off
- Ground chunks with pestle until powdery
- Scrapped into a chilled 50mL conical with the spatula
- Poured in the buffer G2 mix
- Vortexed briefly and placed in incubator genie 10 speed
Noticed ~45 min in that I forgot to add the pro K! Took conical out of the incubator and also noticed that it seemed to be leaking! There was liquid in the incubator, a few mL gone? Quick poured liquid into a new conical and added 500ul of pro k, vortexed and put back into the incubator for another 1.5 hours. This is longer than in the protocol but I was worried about there not being enough time to digest.
Genomic Tip Extraction
Genomic Tip
- Set centrifuge to 4 degrees C
- After incubation, transferred 1mL into each of 8 1.5mL tubes with wide bore pipette tips (some liquid had been lost)
- Centrifuged at 4 degrees C for 10 minutes at 5000 rcf
- Set up tip (resin column) inside a holder over a 50mL conical
- While that was going, added 4mL of buffer to the tip and let drip through to the conical (took the 10 min)
- After centrifugation, added the supernatant from the sample tubes (there was a small brown pellet in each tube) to the tip with wide bore pipette tip
- Started at 11:18 and was finished dripping at 11:31!
- Changed 50mL waste conicals
- Added 7.5mL of buffer QC (wash) and let drip through (10min)
- Warmed 5mL of buffer QF in incubator genie to 50 degrees C
- Repeated wash addition
- Transferred to a different 50mL conical
- Added the 5mL of warmed buffer QF and let drip through
Isopropanol Precipitation of DNA
- Made 6 1.5mL tubes each with 833ul of the elution liquid
- Added 583ul (0.7 volumes) of room temp 100% isopropanol to each tube
- Gently inverted to mix
- Centrifuged at 10,000 rcf for 30 minutes at 1 degree C
- Made fresh 70% EtOH and placed in -20 freezer
- Once finished, looked for pellets. Couldn’t see any, but could be glassy/clear
- One tube at a time, removed supernatant, leaving a little to protect potential pellets
- One tube at a time, added 1mL of cold 70% EtOH and vortexed briefly
- Centrifuged for 10 minutes at 4 degrees C 10,000 rcf
- Removed all of supernatant when finished. This time there was a small tan-ish supernatant visible
- Let tubes air dry ~7 minutes
- Added 100ul of TE bufer to each of the 6 tubes very gently
- Incubated for 1hr at 55 degrees C in the Theremomixer
- Once done, transferred to a shaker overnight 300rpm
QC
- Broad Range Qubit next day
- Flicked tube and took from both top T and bottom B
| Standard 1 | Standard 2 | Sample | Average DNA ng/µl |
|---|---|---|---|
| 151 RFU | 21281 RFU | 1 T | 6.6 |
| - | - | 1 B | 6.56 |
| - | - | 2 T | 6.28 |
| - | - | 2 B | 6.54 |
| - | - | 3 T | 6.5 |
| - | - | 3 B | 7.1 |
| - | - | 4 T | 6.65 |
| - | - | 4 B | 7.4 |
| - | - | 5 T | 6.5 |
| - | - | 5 B | 6.5 |
| - | - | 6 T | too low |
| - | - | 6 B | too low |
Well, the yield is kind of low, or at least not what I was hoping for. If say they’re 6ng/ul and you multiply that by 97ul and 5 tubes, that’s about 3ug of DNA. Not the 25ug we need.
What about the quality?
Genomic DNA screentape
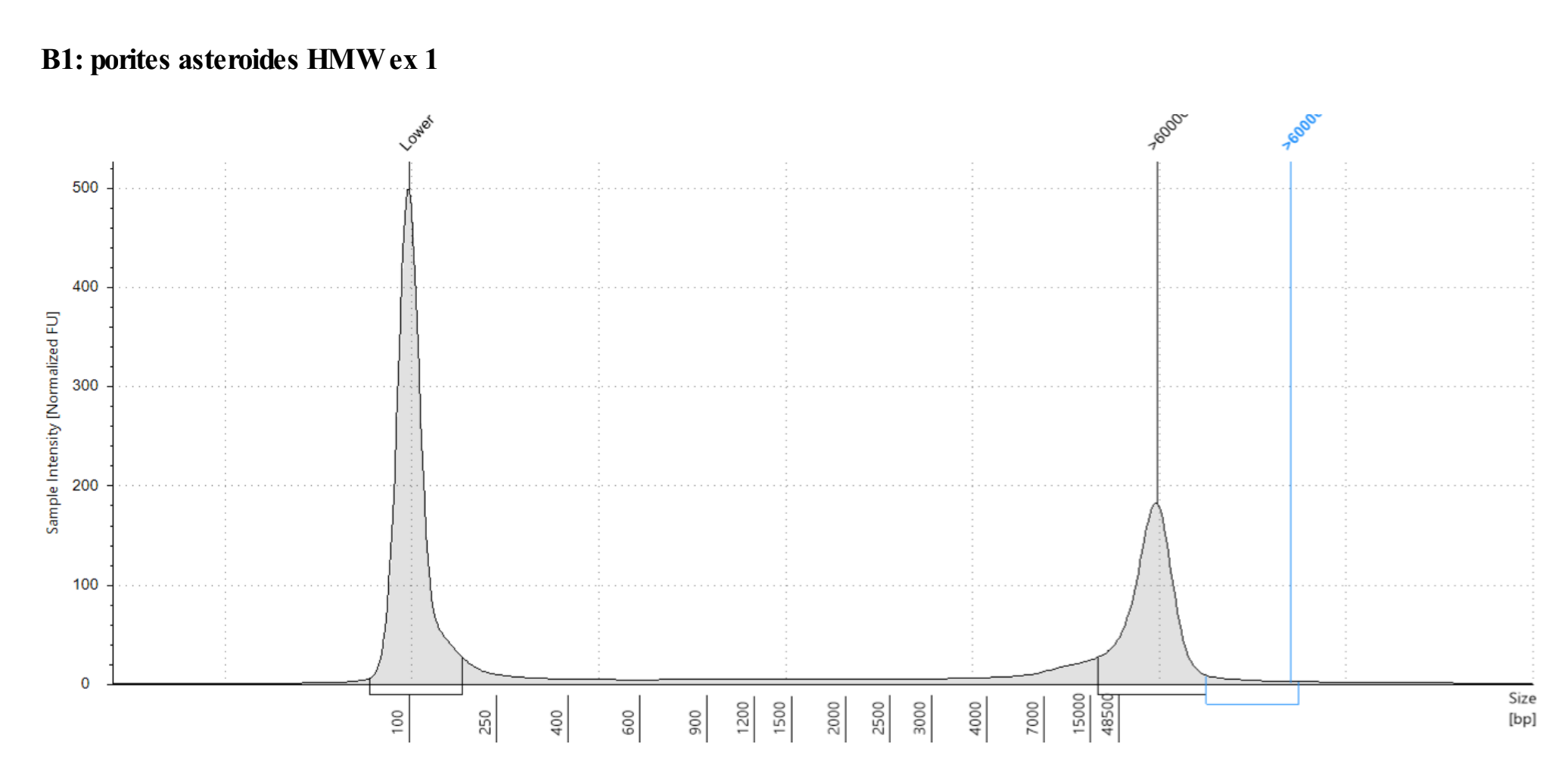
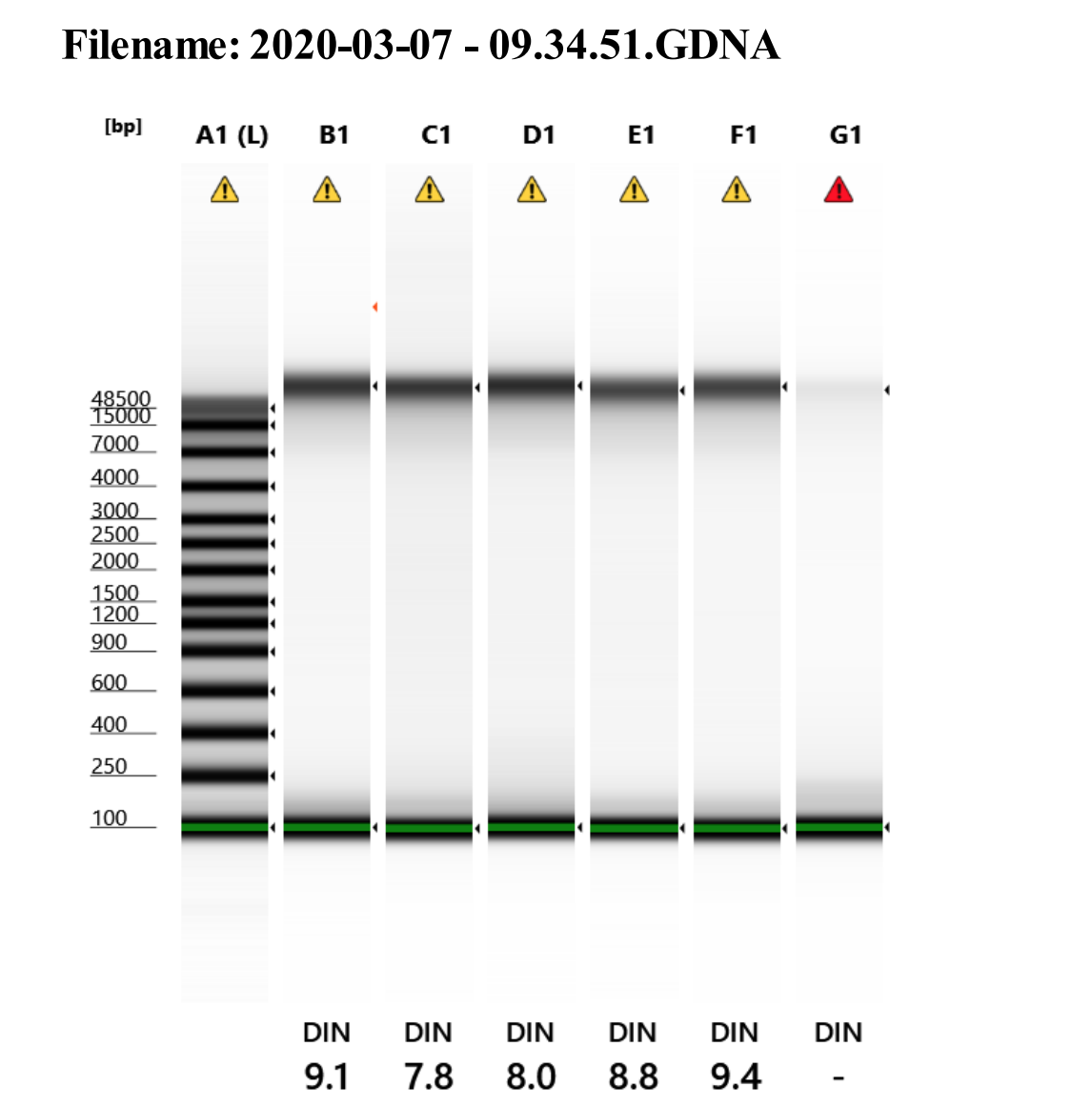
Amazing quality! Just have to extract more!