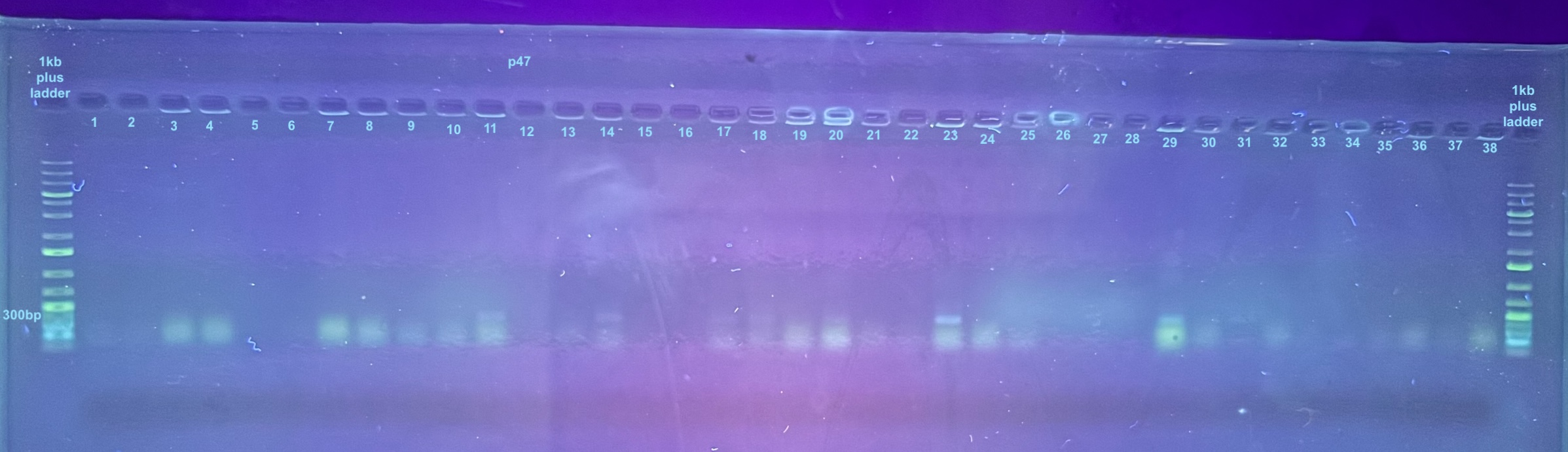
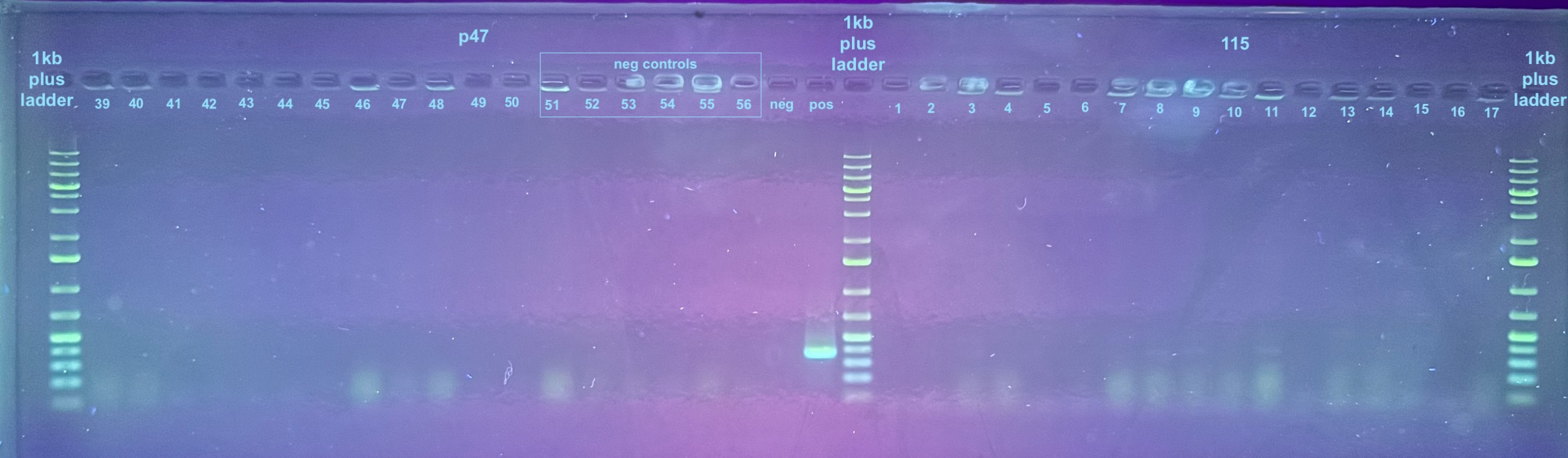
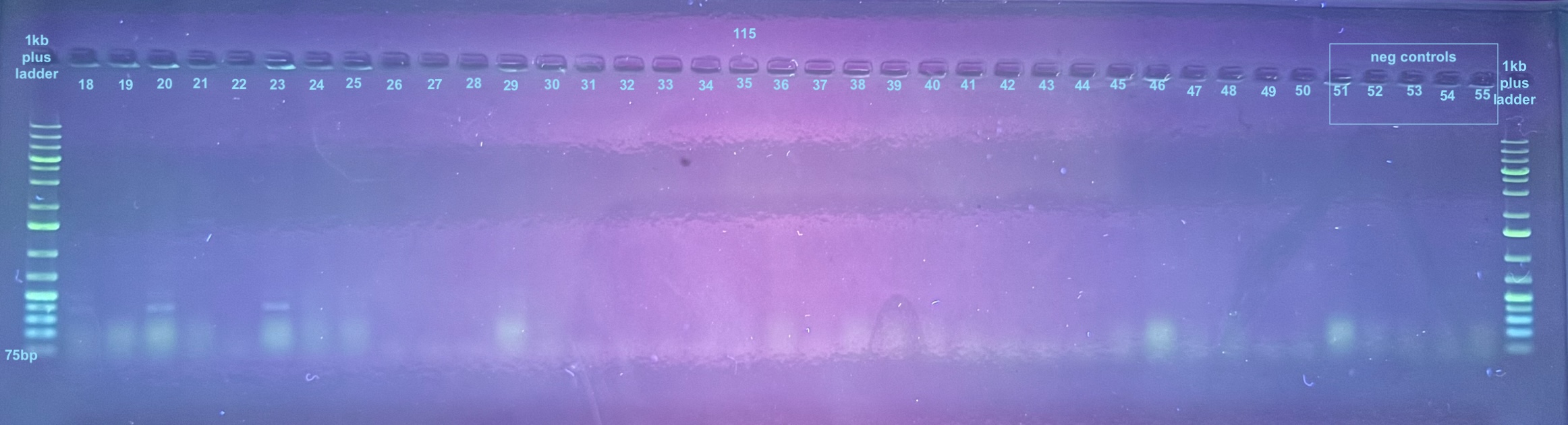
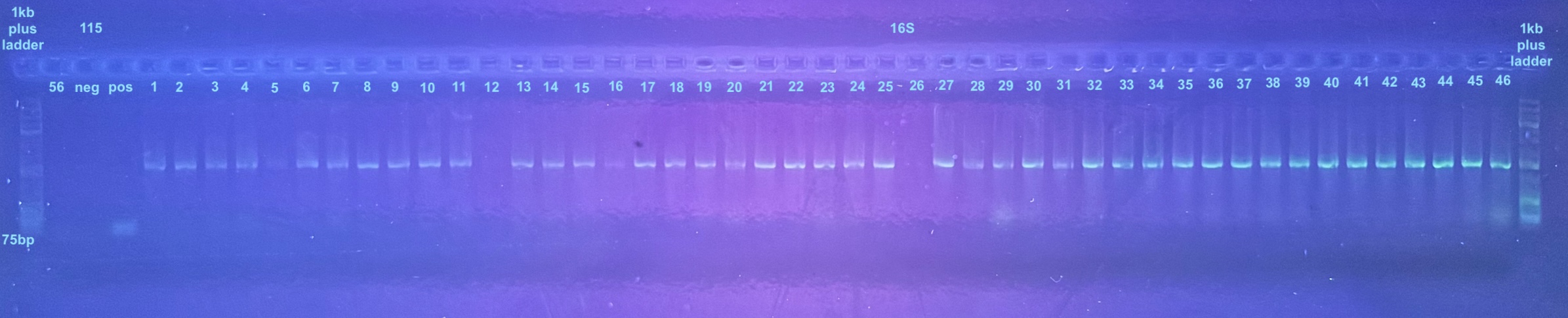
First Set of Colony PCR Plates from Electroporation 2 of pSPIN-BAC E. coli with DiNV DNA
I decided to pick 5 colonies from each DiNV plate from electroporation 2 and 3 colonies from the control plates. The plates took longer to grow at 30C, they were left in the incubator until ~9pm on Tuesday night (had been put in 1-2pm on Monday), and after that they were put in the fridge until the the next morning.
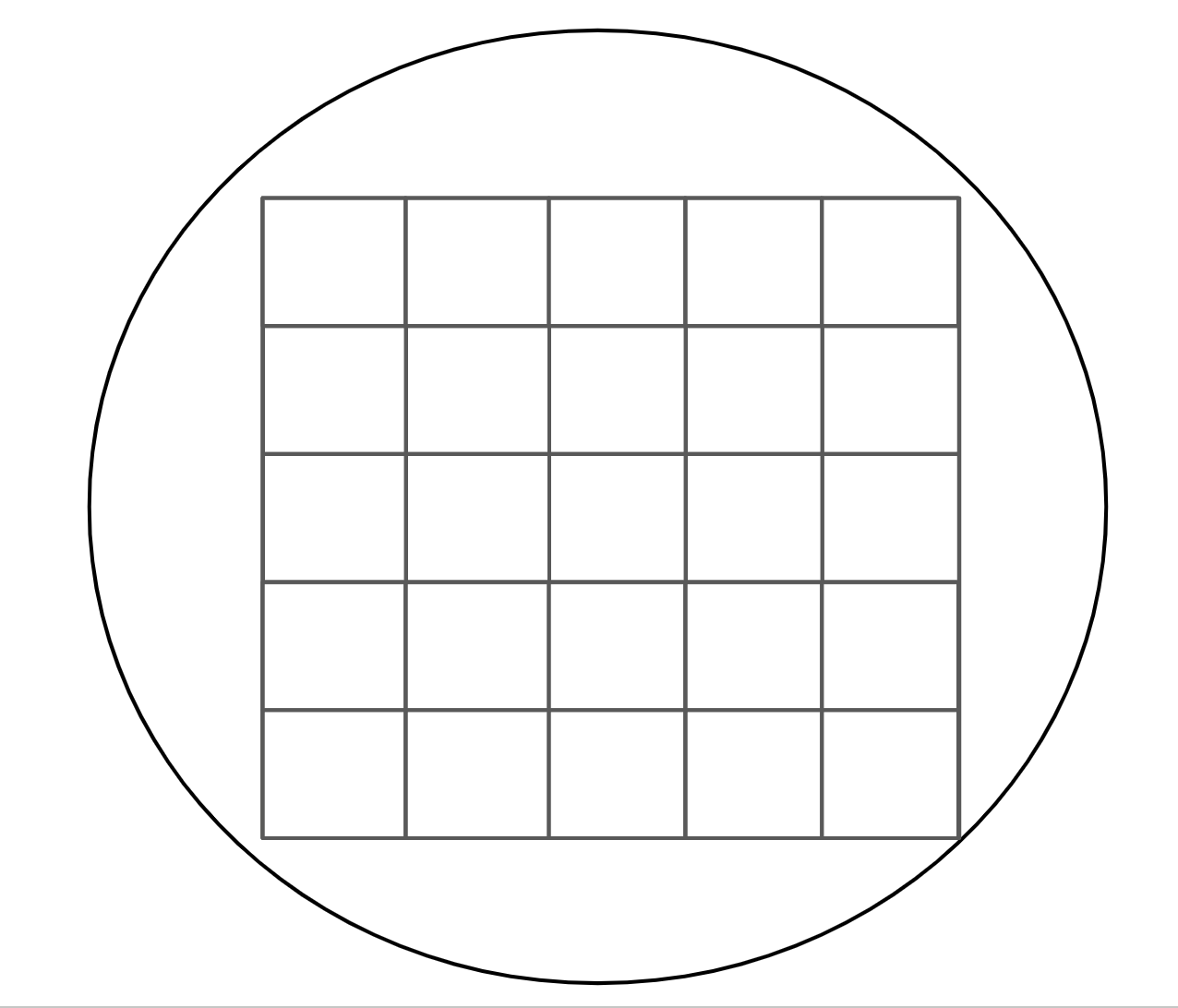
I prepared 56 strip tubes with 5ul of molecular grade water. I also prepared 2 LB plates with chloramphenicol (20ul of 50% stock chlor was spread on each plate and let soak in and warm for ~30 min before use) to keep the colonies on. The plates had a grid layout with 25 spots per plate:
For each colony of the DiNV plates (not the control plates) I picked a colony with a new pipette tip, dipped it on the plate square, and then dipped and spun it in the strip tube for that colony. This way I could keep growing each colony. The plates were left to grow overnight and the put in the fridge to store.
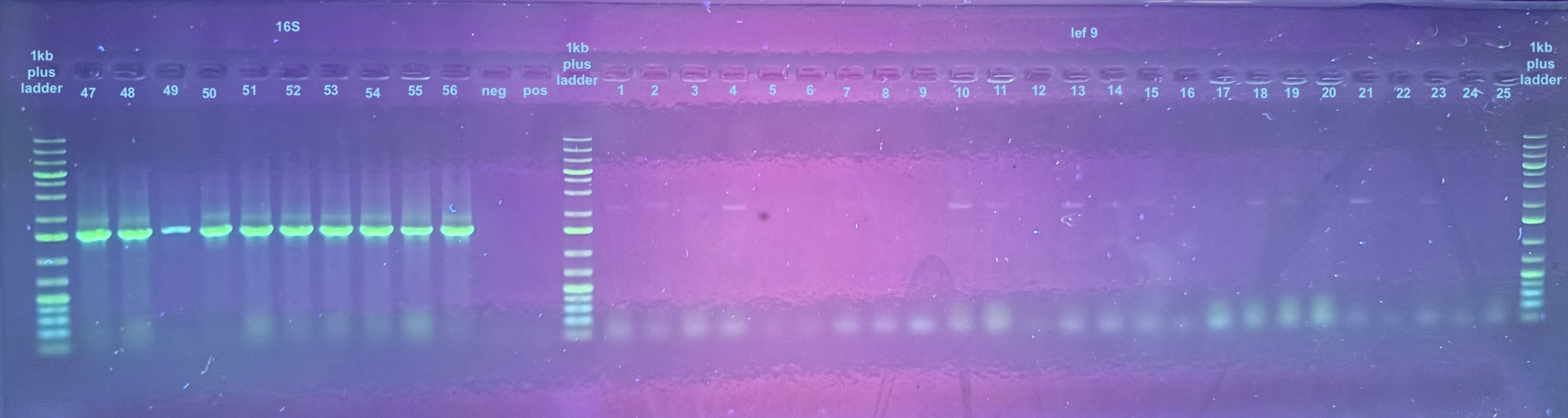
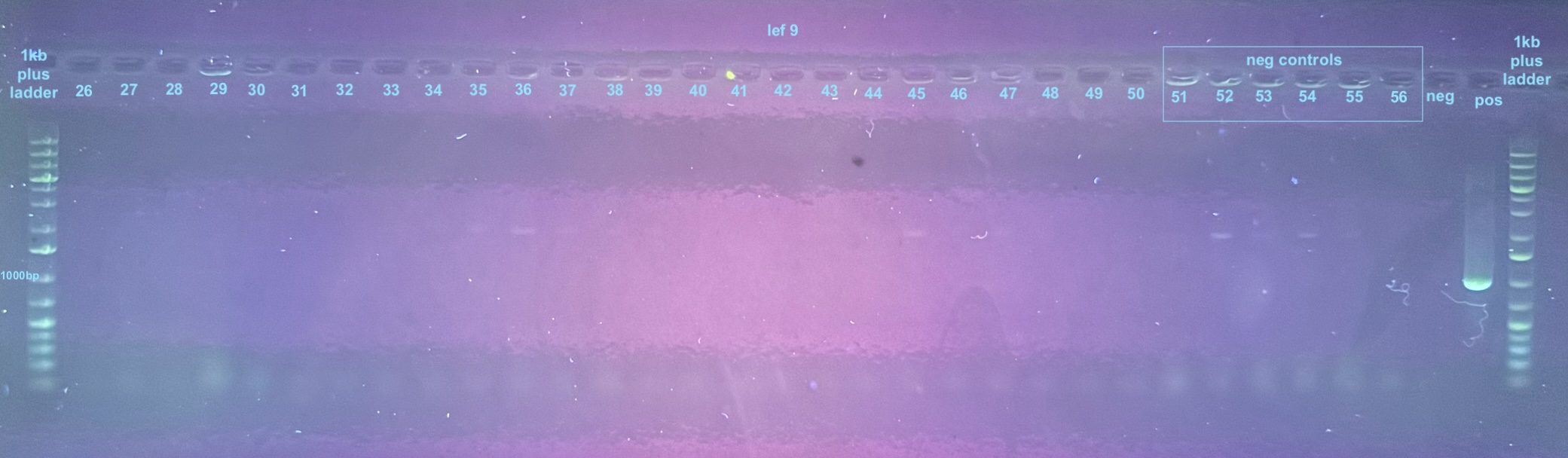
Colonies 1-25 were from the DiNV-1 plates. Colonies 25-50 were from the DiNV-2 plates. Colonies 51-53 were Neg 1 colonies, and colonies 54-56 were neg 2 colonies. All information about the colonies can be found here.
All colonies were then run through 4 PCR primers separately and ran out on gels and products scored. PCR followed the general PCR protocol.
Mastermix volumes are shown here:
| Reagent | p47 | 115 | 16S | lef9 |
|---|---|---|---|---|
| GoTaq | 305ul | 305ul | 305ul | 305ul |
| F primer | 15.25ul | 15.25ul | 15.25ul | 15.25ul |
| R primer | 15.25ul | 15.25ul | 15.25ul | 15.25ul |
| molecular grade water | 213.5ul | 213.5ul | 213.5ul | 213.5ul |
6 separate gels were run because of issues with staining on double laned gels: