Checking for Presence of DiNV in Stock D. innubila Flies from 0-7 Days Post-Emergence with DNA Extractions and PCRs
Previously I had checked the stock D. innubila for presence of DiNV, however all these flies were 0-3 days post-emergence. It could be that DiNV in my stocks shows up when the flies are older. To test this, I took some flies just after emerging (0-3 days old max) and separated them into vials, then froze the vial each day for 5 days. Flies were moved from the vial to 1.5mL tubes with forceps that were dipped in 95% ethanol between each fly. Information on the samples are in the table below:
| tube_number | day_frozen | age |
|---|---|---|
| 1 | 20230721 | 0-3 days |
| 2 | 20230721 | 0-3 days |
| 3 | 20230721 | 0-3 days |
| 4 | 20230721 | 0-3 days |
| 5 | 20230722 | 1-4 days |
| 6 | 20230722 | 1-4 days |
| 7 | 20230722 | 1-4 days |
| 8 | 20230722 | 1-4 days |
| 9 | 20230723 | 2-5 days |
| 10 | 20230723 | 2-5 days |
| 11 | 20230723 | 2-5 days |
| 12 | 20230723 | 2-5 days |
| 13 | 20230724 | 3-6 days |
| 14 | 20230724 | 3-6 days |
| 15 | 20230724 | 3-6 days |
| 16 | 20230724 | 3-6 days |
| 17 | 20230725 | 4-7 days |
| 18 | 20230725 | 4-7 days |
| 19 | 20230725 | 4-7 days |
20230727 DNA extraction
DNA was extracted from each fly using the general single fly extraction protocol, with a few changes. Notably, filter tips were used in all steps, and instead of using the motorized pestle grinder to homogenize flies, the pestles were used to homogenize the flies by hand to avoid any splashing of fly fluid. Additionally all supernatants were pipetted out of tubes instead of poured.
An extraction control sample was made, with an empty tube getting all the extraction steps performed on it.
Information on these samples can be found here
20230728 PCRs
PCRs on CO1, TPI, p47, and 115 were done accordingly to the general PCR protocol with the primers, and their information can be found here. The p47 and 115 samples were amplified in 30 cycles. CO1 and TPI primers were used in the same tube, while the p47 and 115 primers were used in separate reaction tubes. Each primer set had a negative control, however a positive control was not used. The extraction control was used in all PCRs as a check of the extraction process.
After the PCRs, a 2% gel was run in the largest gel box: 3.3g agarose, 165mL 1X TAE, and 2ul of Midori stain. The gel was ran for 35 minutes at 90V.
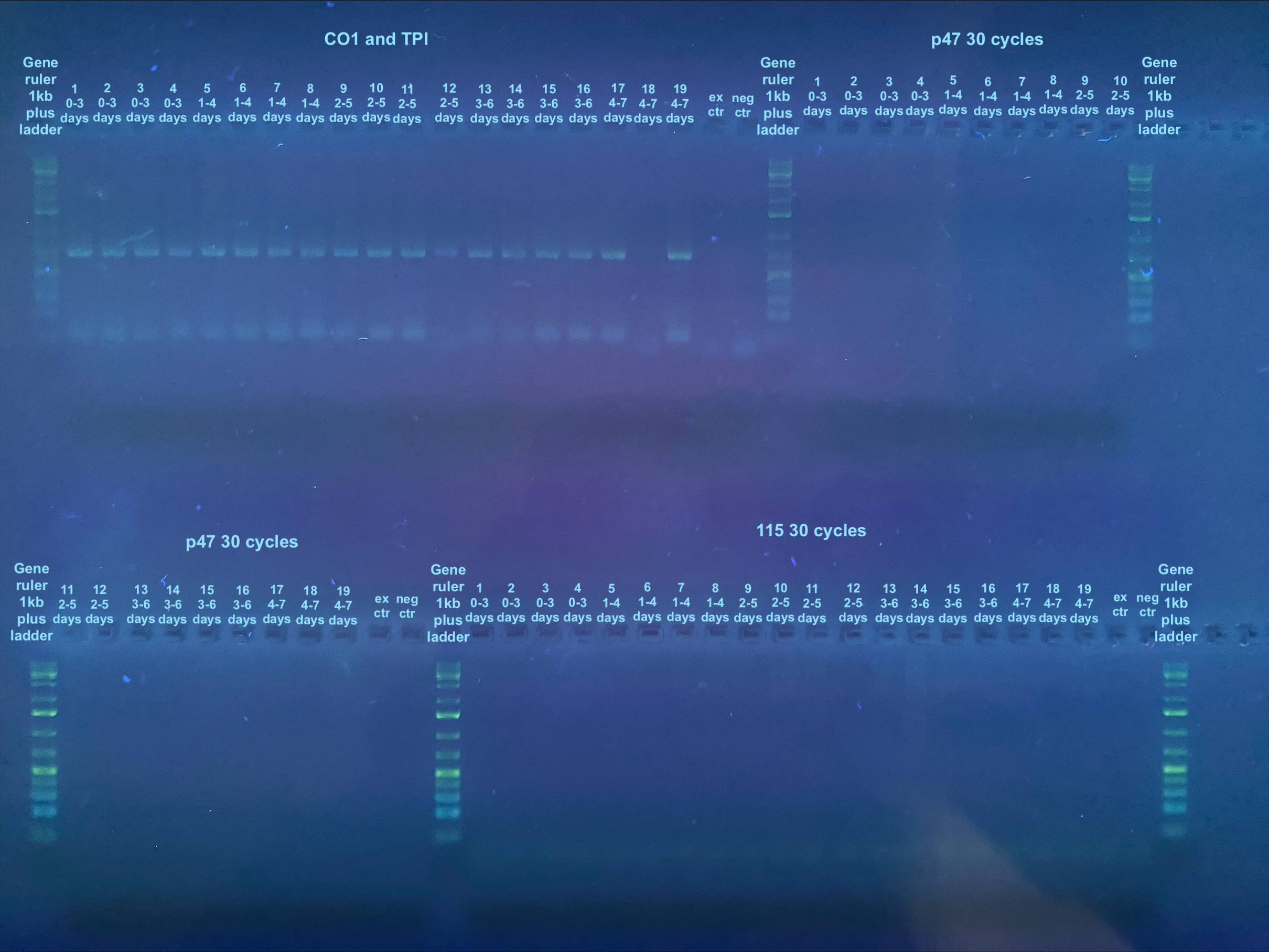
Information on determining bands can be found here. Looks like all samples are negative for virus. However there is one sample that did not extract well and nothing showed up for the control DNA PCRs. Additionally, nothing showed up for the control extraction. However, because I didn’t do a positive control for the virus samples, technically I don’t know if the PCRs didn’t work.