Cell Culture Inventory And Plating 3.1 Cells for Transfection on 5-23
Cell Culture Inventory
3/22 ovaries
- 1 flask
- Flask full of debris
- I don’t see any alive cells
- Flask was thrown out
4/3 and 4/11 primaries
- 2 flasks for each date
- Supernatant flask is very full of debris
- Other flask is mostly full of dead cells, doesn’t seem like anything is alive in either flask
- Flasks were thrown out
1.1 innubila P2
- 1 flask
- A couple of good areas of growing cells
- Want to fluid change
- This flask may have enough cells to scrape and try re-plating again
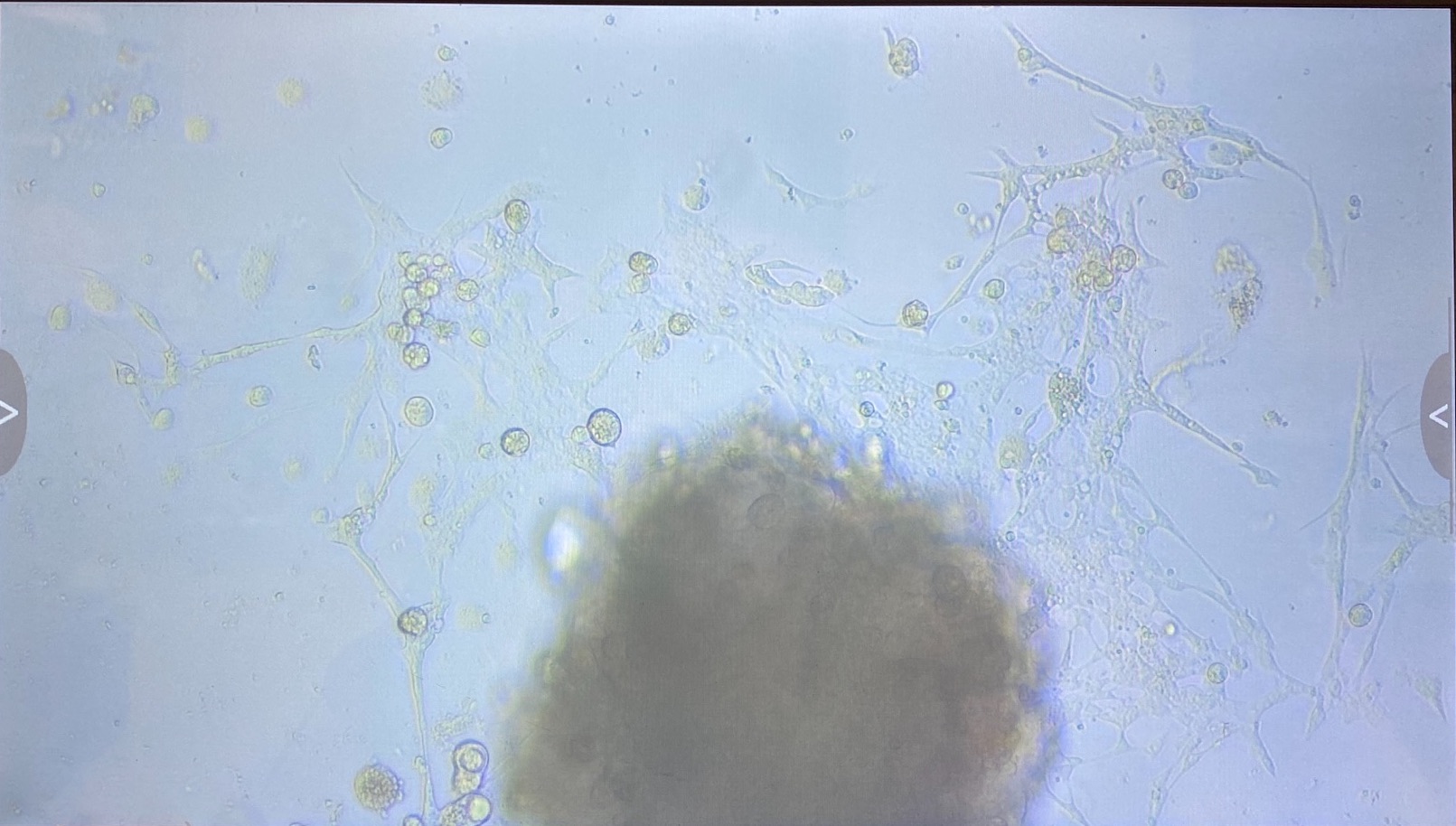
1.2 innubila P2
- 1 flask
- Some small clumps of cells growing ok
- Want to fluid change
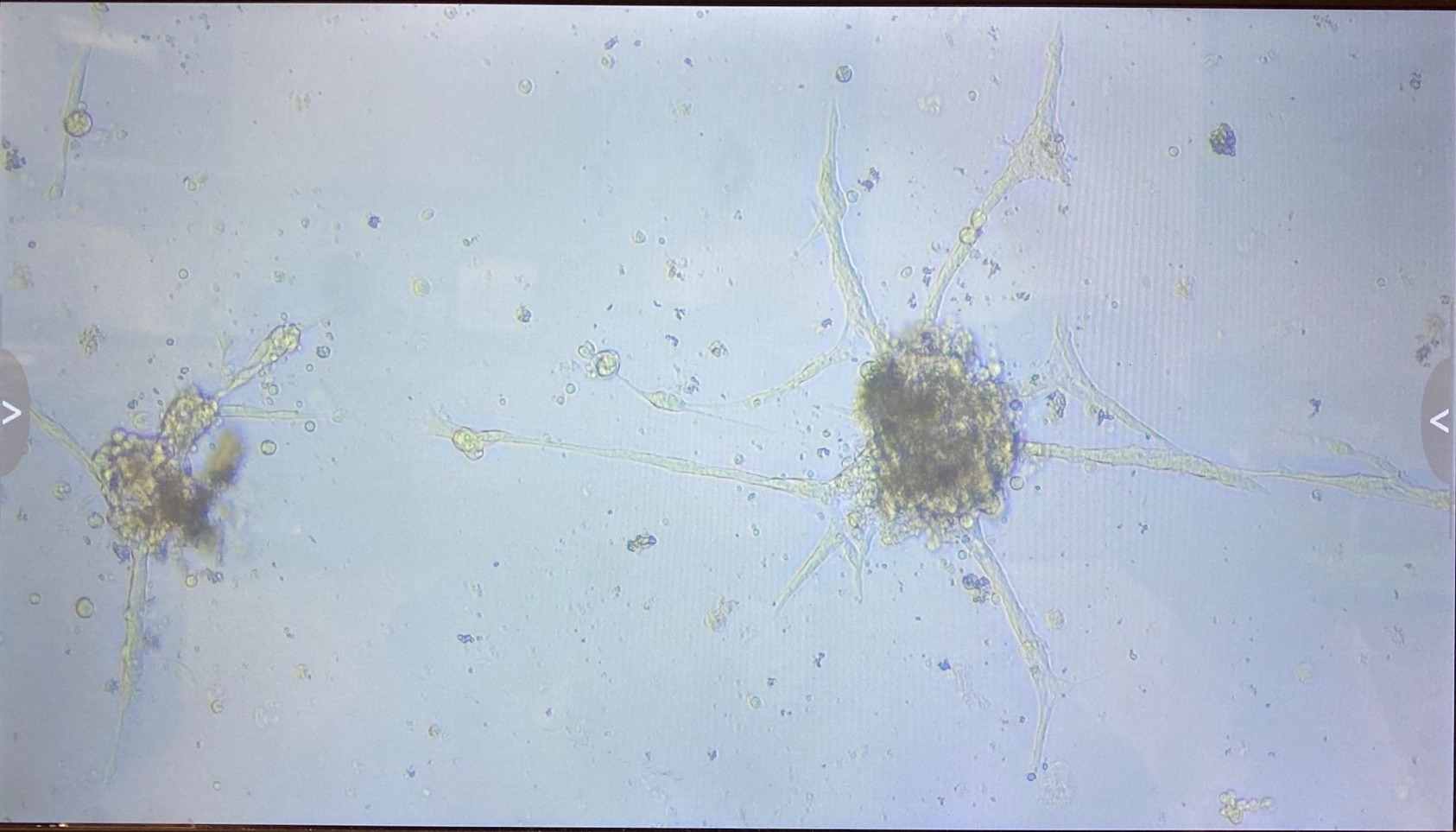
1.2.1 innubila P3
- 1 flask
- Some small clumps of cells growing ok
- Want to fluid change

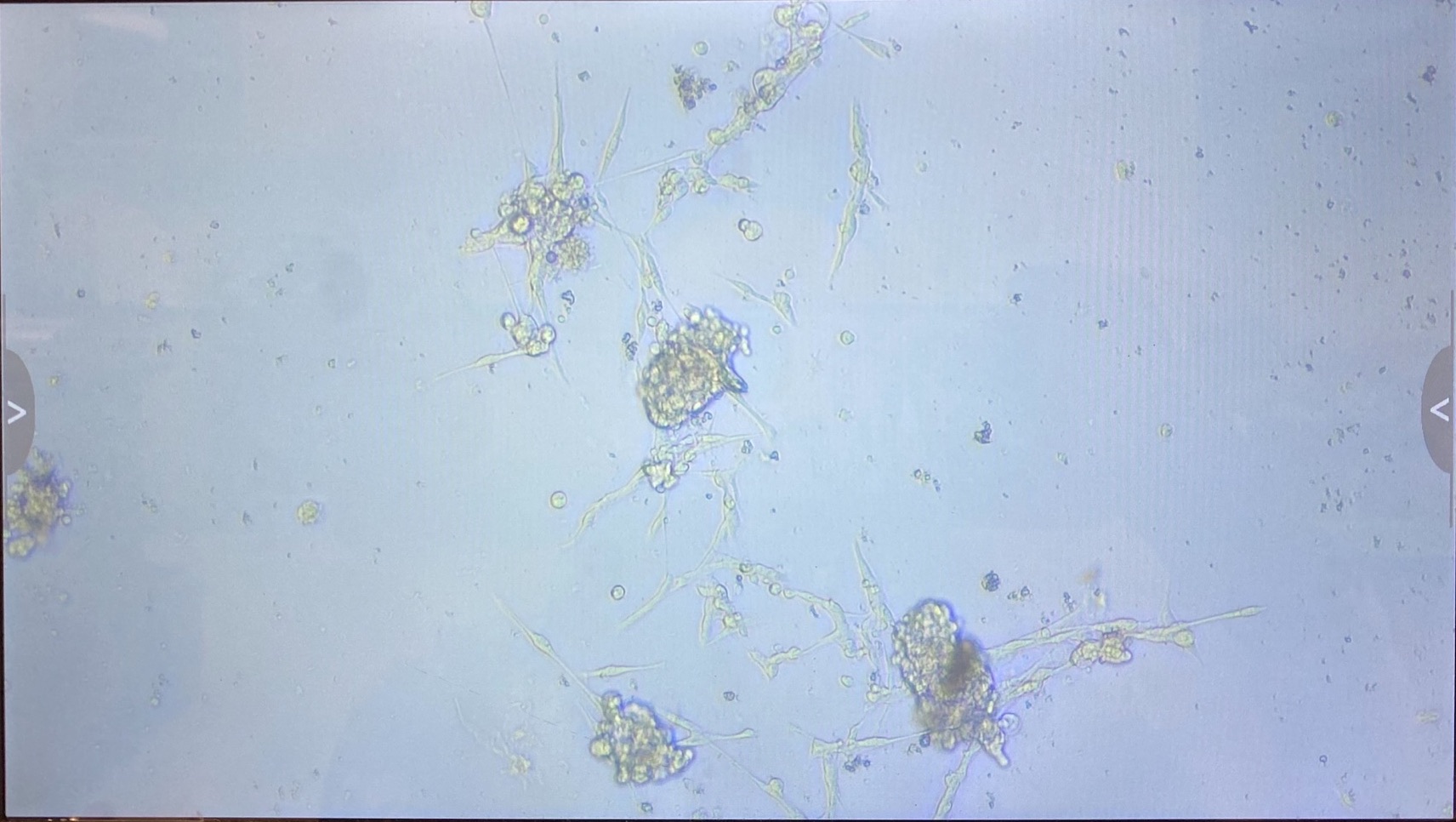
1.3 innubila P2
- 1 flask
- Has a good number of good looking areas of cells, but this flask also has more precipitate than other flasks
- Want to fluid change
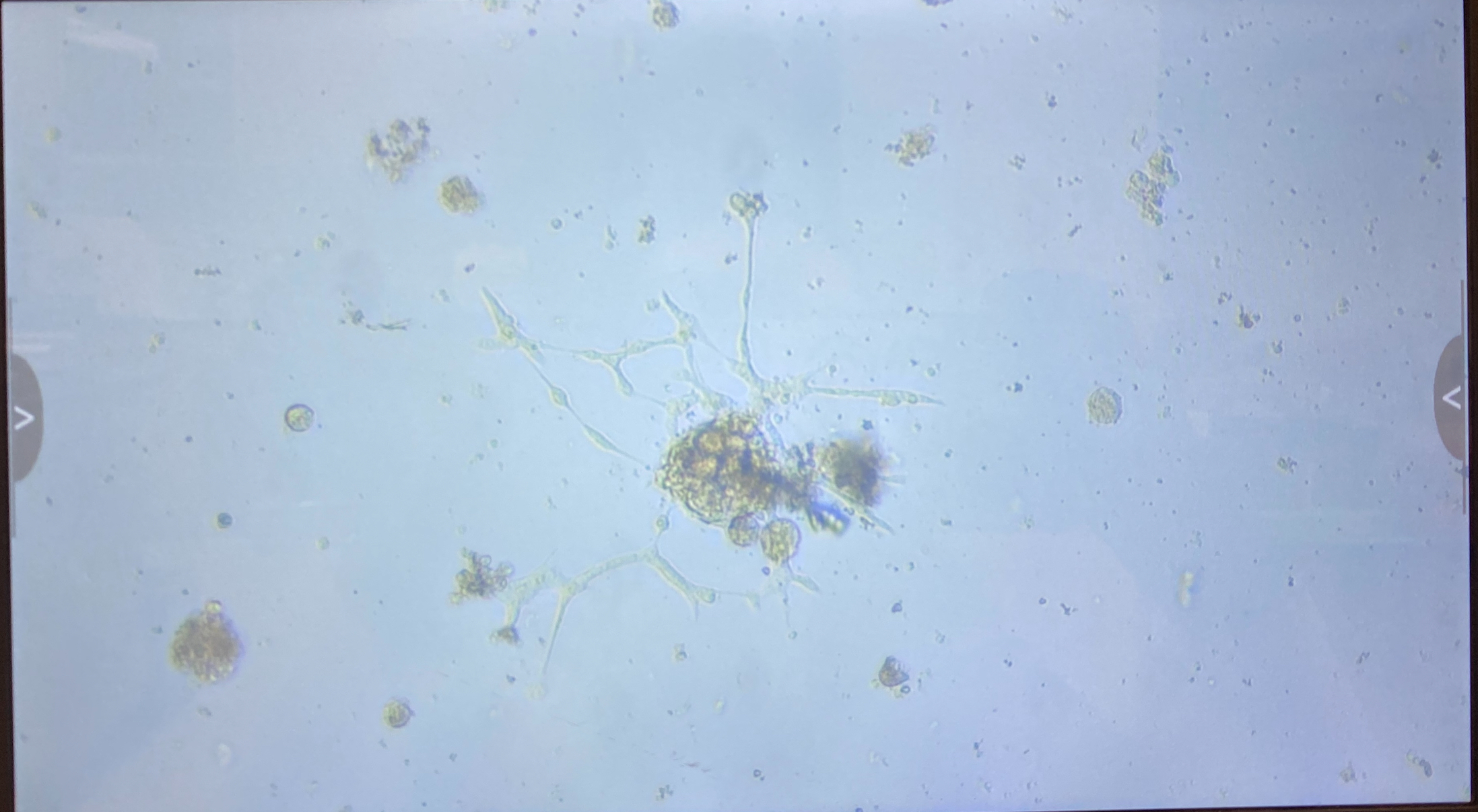
1.3.1 innubila P3
- 1 flask
- Some small clumps of cells growing ok
- Want to fluid change
1.4 innubila P2
- 1 flask
- Some small clumps of cells growing ok
- Want to fluid change

1.5 innubila P2
- 1 flask
- Only one small area of growing out cells…
- Want to fluid change

1 innubila P1 from 1/12
- 1 flask
- Basically this flask is entirely full of precipitate
- Flask was thrown out
G and C innubila primaries
- 3 flasks
- All are very full of precipitate
- Flasks were thrown out
E, D, and G.1 innubila Primaries
- 3 flasks
- Many dead cells and precipitate with nothing growning
- Flasks were thrown out
Passing 14091 Myd88 cells
- Cell scraped p26 flask
- Used 1mL to seed 2 new flasks
- Each flask was suspended with 5mL of 10% FBS Schnedier’s medium
Fluid Exchanges
- Removed 3mL from each flask
- Added back in 3mL of 20% FBS, 4% mushroom broth Schneider’s medium
- Flasks:
- 1.1
- 1.2
- 1.2.1
- 1.3
- 1.3.1
- 1.4
- 1.5
Plating 3.1 Cells in Plate 9
- Wanting to do another transfection experiment, so I want to use the 3.1 cells in a 12 well plate
- Took the 3.1 P3 flask and poured of the medium
- Added 5mL trypsin and immediately poured off
- Added 5mL trypsin and waited ~5-10 minutes for the cells to release from the bottom of the flask
- Sucked up the liquid and put it into a 15mL tube
- Centrifuged the tube for 5 min at 200rpm
- Removed the trypsin
- Resuspened the cells in 10mL of 10% FBS, 4% mushroom Schneider’s medium
- Ran the cell fluid through a 70um filter
- Resupended the cells stuck on the filter in 10mL 10% FBS, 4% mushroom Schneider’s medium and added that to 2 new flasks for a passage 4
- For the 10mL fluid that went through the strainer, 1.5mL of this was added to wells A2, B2, C2, A3, B3, and C3 of plate 9 (see layout)
| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| A | S2 control | 3.1 DinnDiNV control, 100ul schneider’s medium | 3.1 DinnDiNV control, 100ul schneider’s medium | X | ||||
| B | S2 2020 1ul reagent | 3.1 DinnDiNV, 1ul 2020 reagent, 1ul pAc5 | 3.1 DinnDiNV, 1ul 2020 reagent, 1ul pAc5 | X | ||||
| C | S2 2020 1ul reagent | 3.1 DinnDiNV, 1ul 2020 reagent, 1ul pAc5 | 3.1 DinnDiNV, 1ul 2020 reagent, 1ul pAc5 | X | ||||
Passing 4.3 DinnDiNV cells
- I make sure to finish all of my other cell work before starting on these cells because they are persistently infected with DiNV
- Going to use trypsin to pass these cells
- Poured off the medium
- Added 5mL trypsin then poured it off
- Added another 5mL trypsin and waited ~5-10 min for the cells to release from the flask
- Added the fluid to a 15mL tube and centrifuged for 5 min at 200rpm
- Removed the supernatant from the cell pellet
- Resuspended the cells in 12mL 20% FBS, 4% mushroom Schneider’s medium
- Separated out that fluid into 2 new flasks for passage 2