Counting and Plating S2 Cells and Adding 3.1 Cells to Plate 8 For Transfection
I left the 3.1 and 3.0 DinnDiNV cells that I tried my best to de-clump and plated in the 12 well plate on 4/27 for over a week, and two columns (the 3.0 cells that were scraped) just had not grown very much. I thought that there had just been too few clumps of cells to start growing out properly in those wells.
Plate layout:
| PLATE 8 | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| A | S2 | 3.0 top fluid, strained | 3.0 bottom fluid, strained | 3.1 top fluid, strained | ||||
| B | S2 | 3.0 top fluid, strained | 3.0 bottom fluid, strained | 3.1 top fluid, strained | ||||
| C | S2 | 3.0 top fluid, strained | 3.0 bottom fluid, strained | 3.1 top fluid, strained | ||||
Here are the photos of every well on the 8th of May:
A2
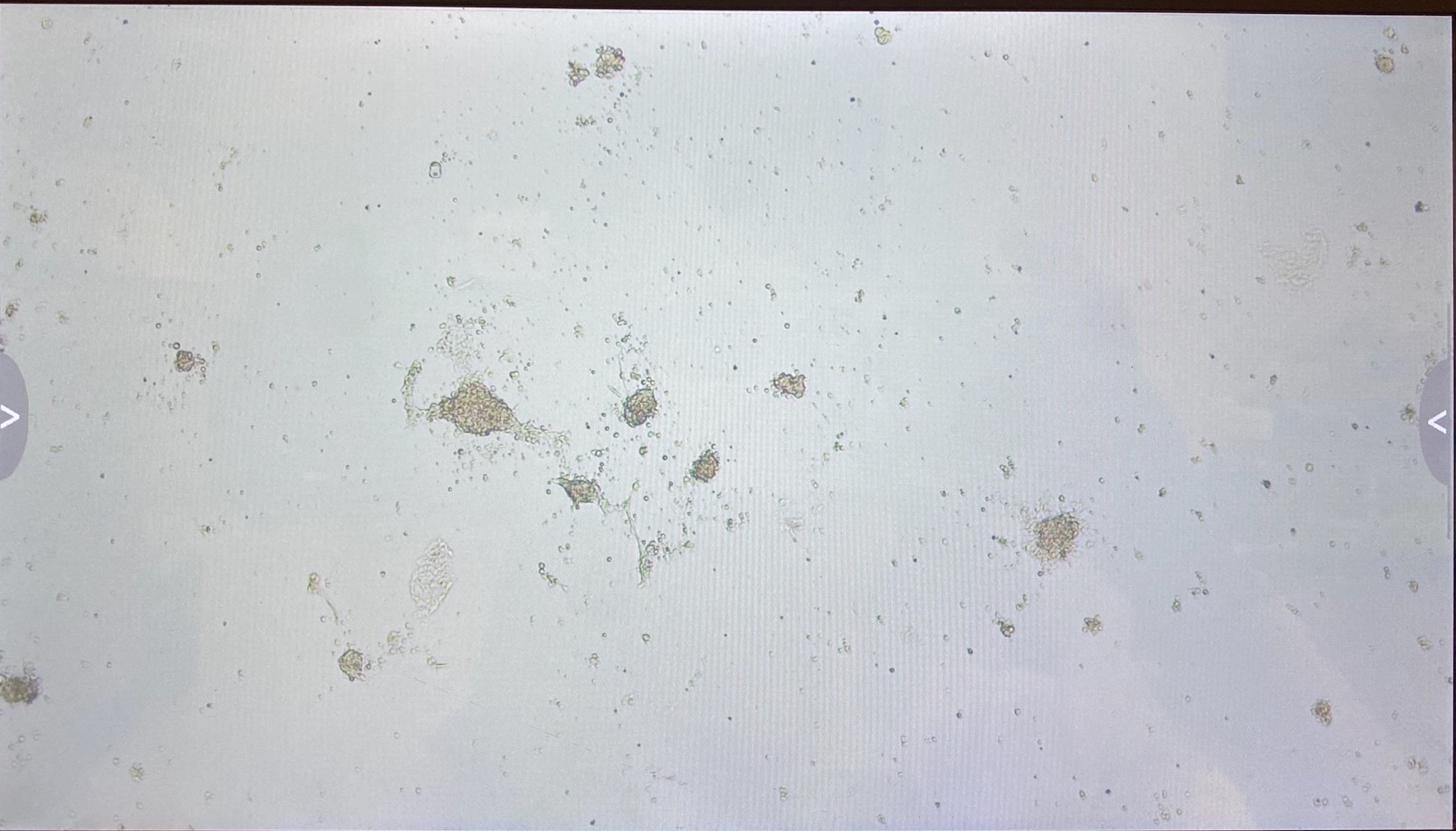 B2
B2
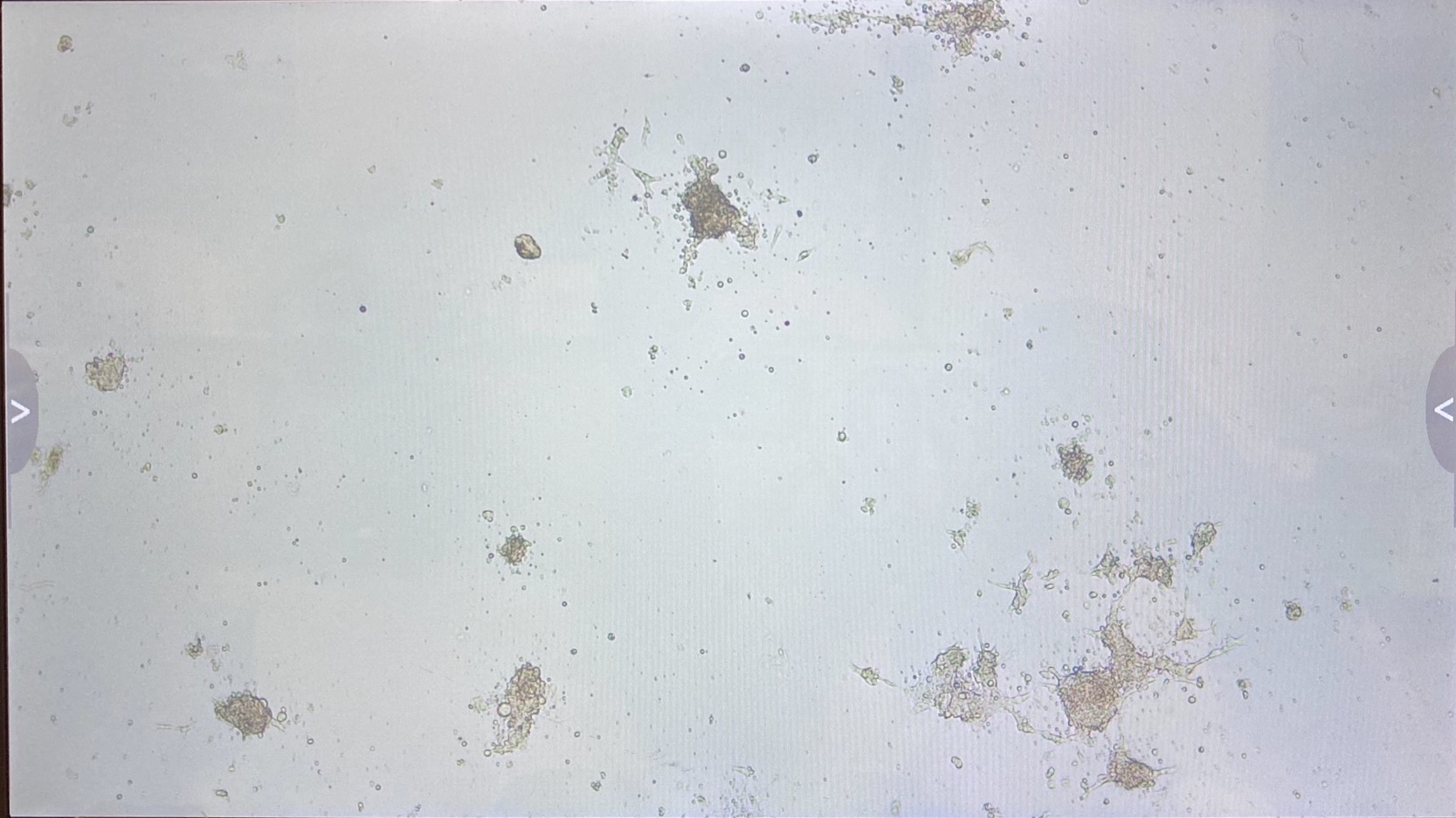 C2
C2
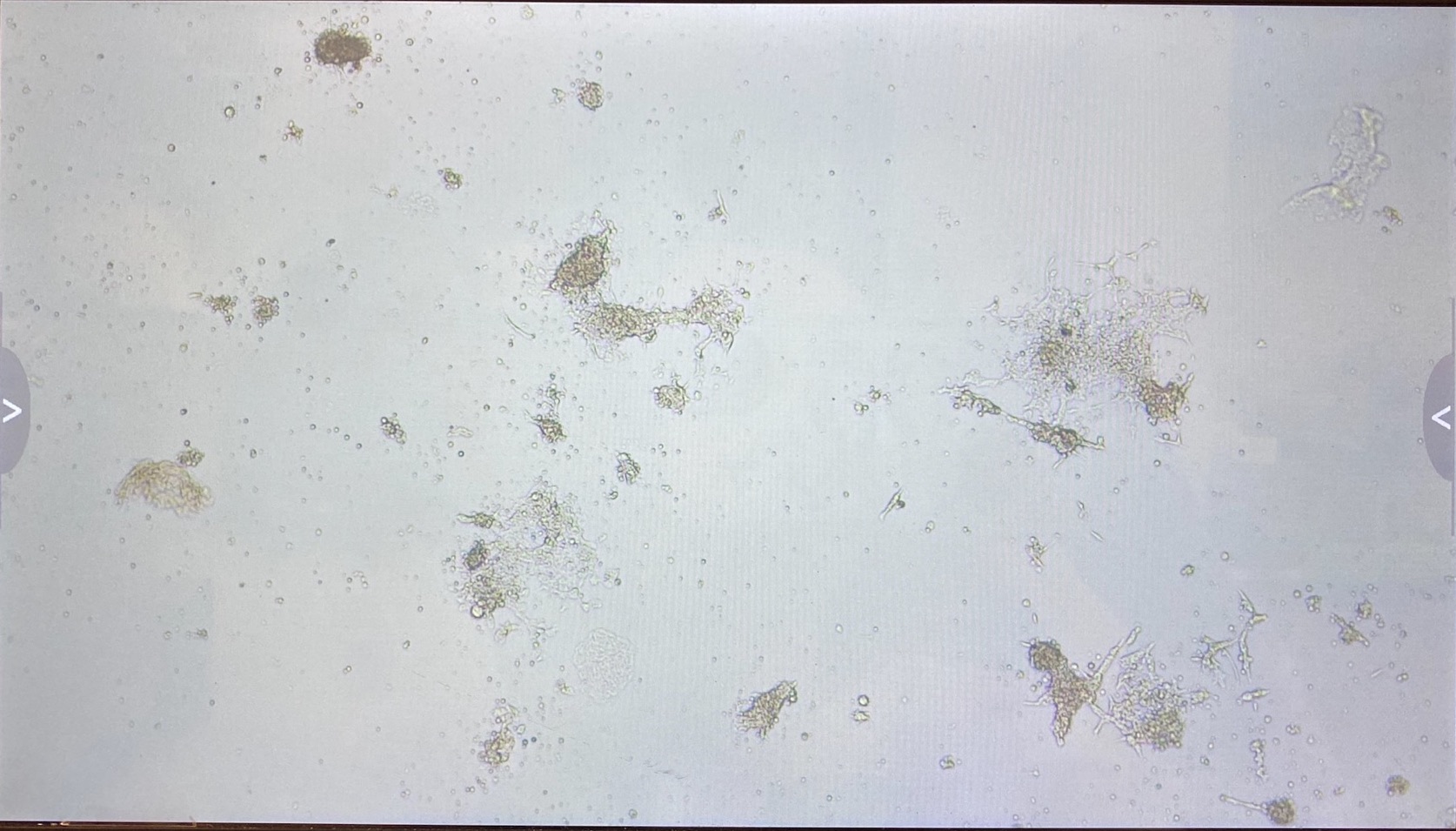 A3
A3
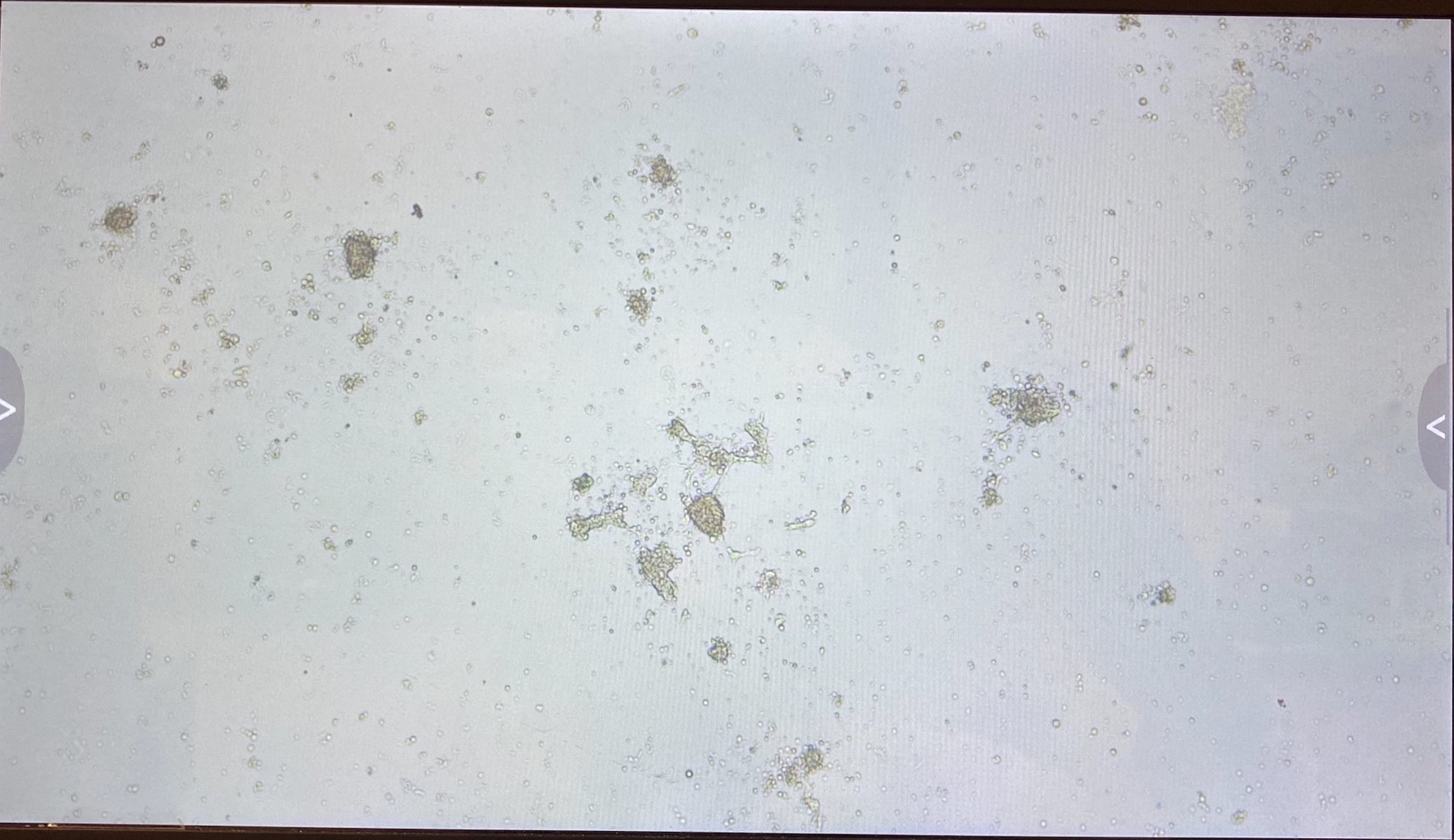 B3
B3
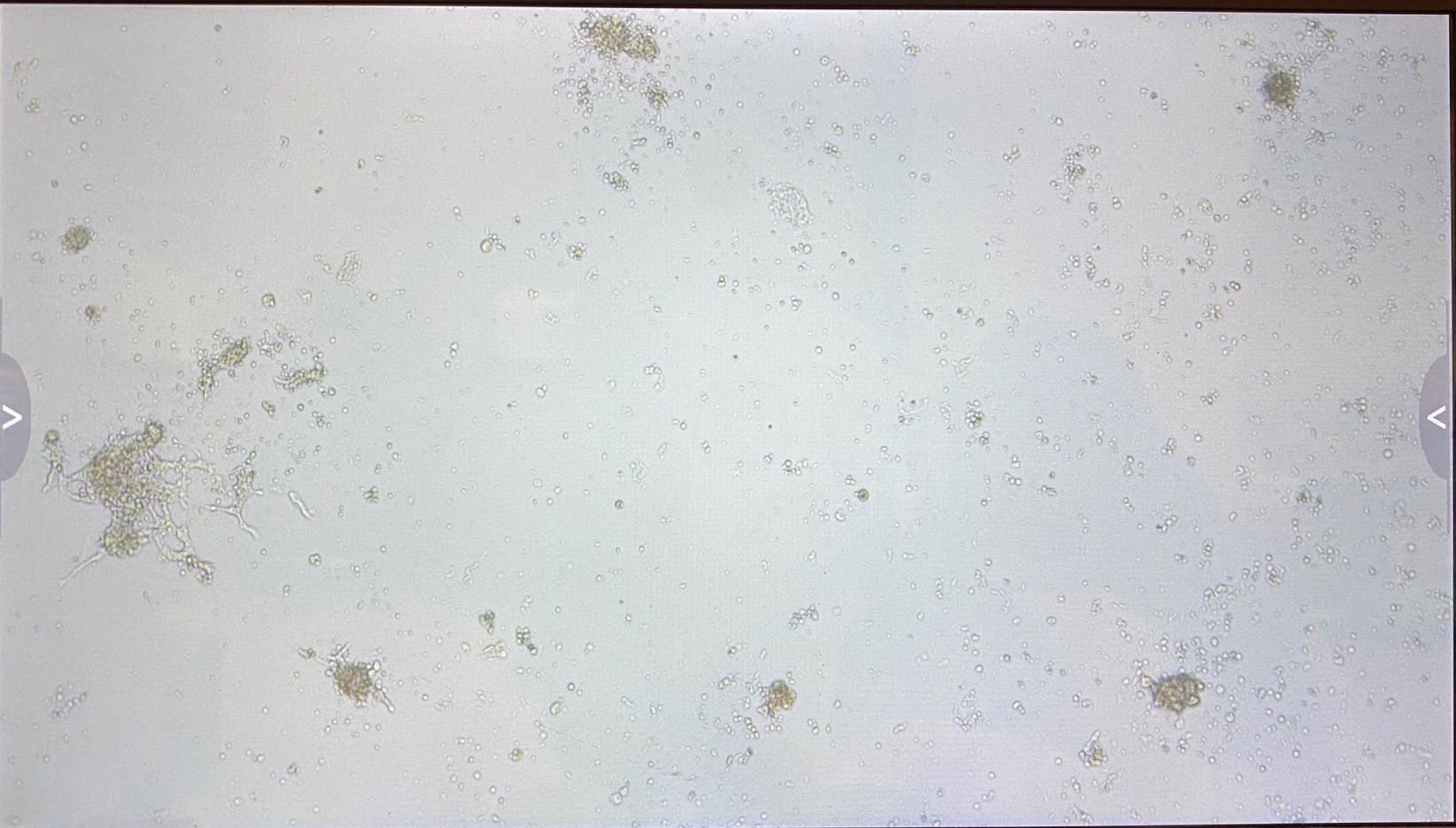 C3
C3
 A4
A4
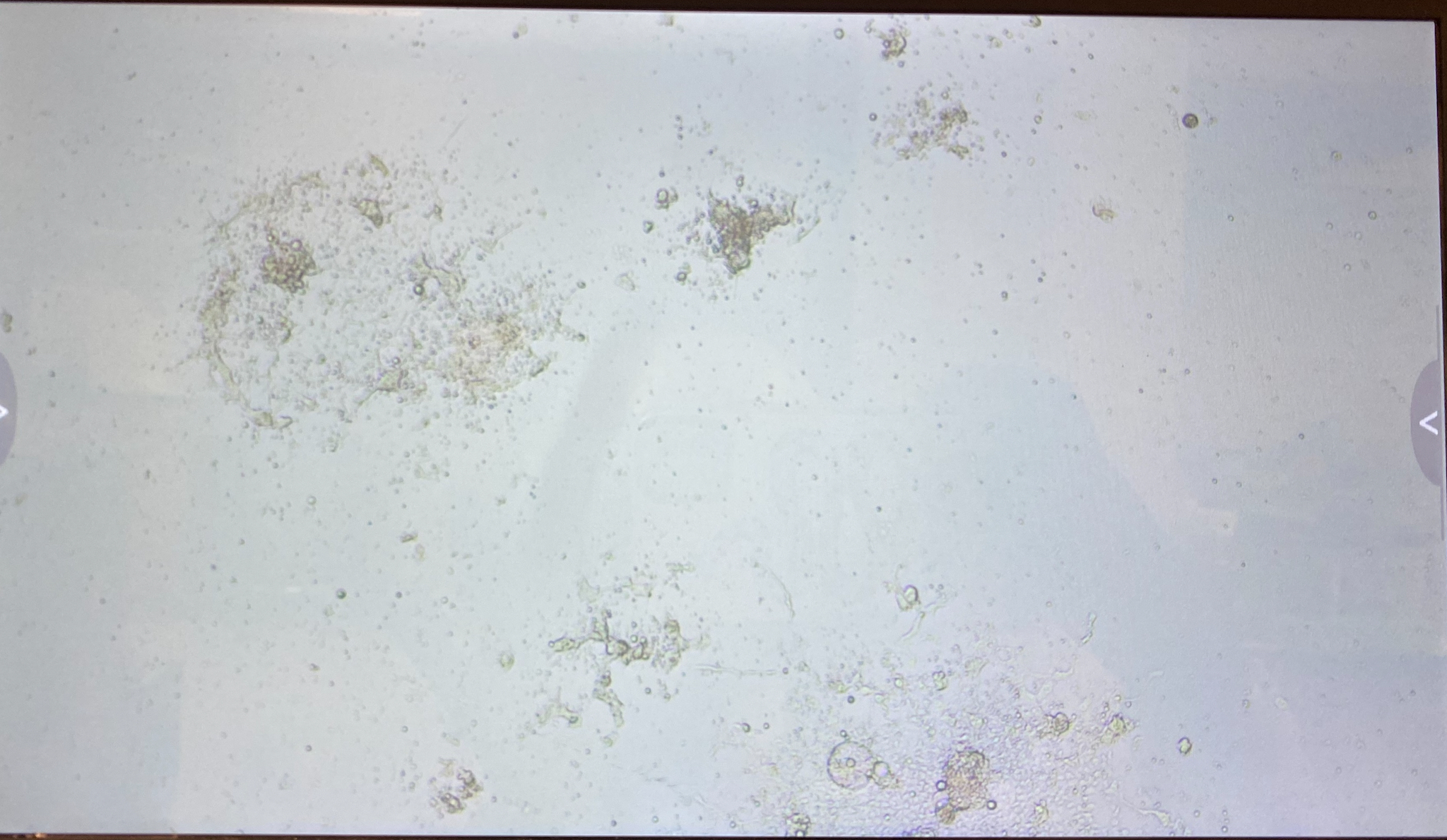 B4
B4
 C4
C4
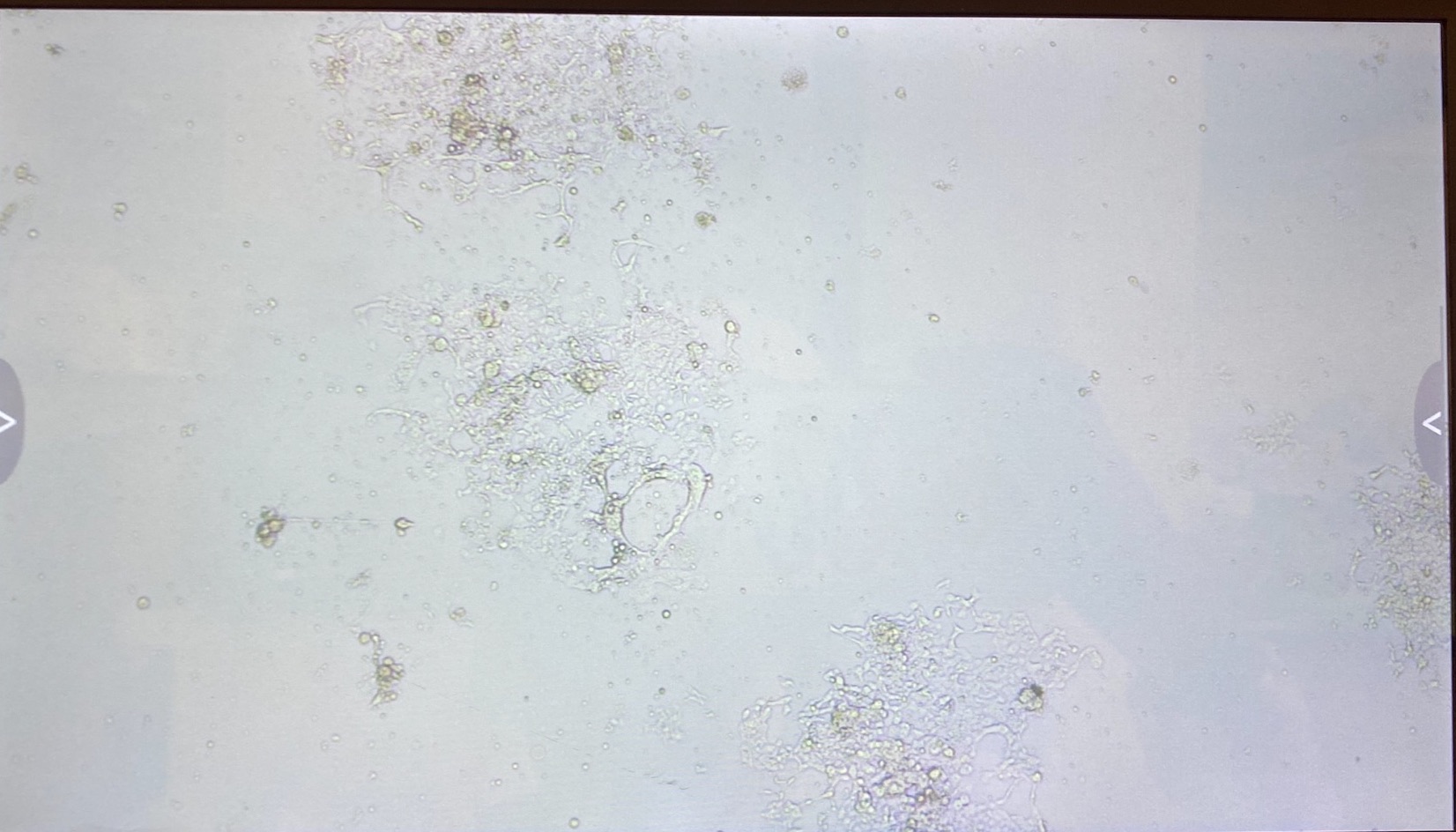
The 3.1 cell fluid wells have a lot more growth, so I decided to keep those wells the same. I had 3.1 top of filter cells from 4/27 that had been growing in a flask since then, that I decided to trypsinize and add to the wells that hadn’t been doing good.
Adding more DinnDiNV cells
- Poured off the medium from the 4/27 3.1 flask
- Added 3mL trypsin, rinsed, and poured off
- Added 5mL trypsin and waited ~5 minutes until all the cells had come off the bottom of the flask
- Took the fluid and put it into a 15mL tube
- Centrifuged the tube for 2 minutes at 200rpm
- Removed all the supernatant from the pellet of cells
- Resuspended the cells in 6mL 20% FBS Schneider’s medium with 4% mushroom
- Ran those 6mL through a 70um cell strainer
- Resuspended the top of the filter captured cells in 5mL of 20% FBS Schneider’s medium with 4% mushroom and plated that in a new flask for passage 3 of those cells
- The 6mL that had passed through the 70um filter was distributed throughout the center 6 wells on plate 8, columns 2 and 3
Counting and plating S2 cells
- I decided to plate the S2 at a slightly lower number than I have done previously, 100,000 cells per mL instead of 150,000 cells per mL, because I am not able to count the DinnDiNV cells and I guess that there are less of them
- Took an old flask of cells and removed 3mL from the fluid (many cells in the supernatant) and put it in a 15mL tube
- Added 3mL 10% FBS Schneider’s medium to the tube and mixed
- Took 20ul from the cell fluid in the tube and put it in a hemocytometer to count the number of cells:
| section | quadrant 1 | quadrant 2 | quadrant 3 | quadrant 4 | section average |
|---|---|---|---|---|---|
| 1 | 128 | 118 | 102 | 107 | 114 |
| 2 | 117 | 103 | 91 | 102 | 103 |
- Each section was averaged, and the average of the two sections was taken
- Total S2 average: 109
- The cells per mL is the average * 10^4
- 109 * 10^4 = 1,090,000 cells per mL
- (1.09*10^6 cells/mL)(5mL) = (100,000 cell/mL)(xmL)
- x = 65.4mL medium
- Because I only want 6mL, I divided the xmL and the cell volume 6mL by 10
- 6.54mL 10% FBS medium
- 0.6mL cell resuspention
- This was made, and 1.5mL S2 cell suspension was put in wells A1, B1, and, C1
The plate was put in the incubator to grow overnight