Ringers Solution, D. innubila Dissections, and Primary Cell Generation
20230215 Ringers Solution
- Want to dissect the flies in ringers solution, it is not at the Biostore so I should make it myself
- I used this recipe cut in half to make 500mL
- I used this resource on how to make 1N HCl solution
- I mixed these chemicals in 450mL of diH20:
- 0.165g of Calcium Chloride dihydrate
- 6.8g of Potassium Chloride
- 1.35g of Sodium Chloride
- 0.605g of Tris Base
- Then I checked the pH with pH test strips. The pH is supposed to be ~7.2 and I am supposed to use HCl to titrate it down. This was kind of hard because I got a second brand of test strips (seemed more sensitive) and it have me different info than the other brand.

- Because the other brand said the pH was perfect, I was inclined not to believe it. Plus I check the whatman indicator strips on di-water and it said that pH was 7, which I assumed was right. So I decided to trust the Whatman strips that said the pH was at 10
- water pH:
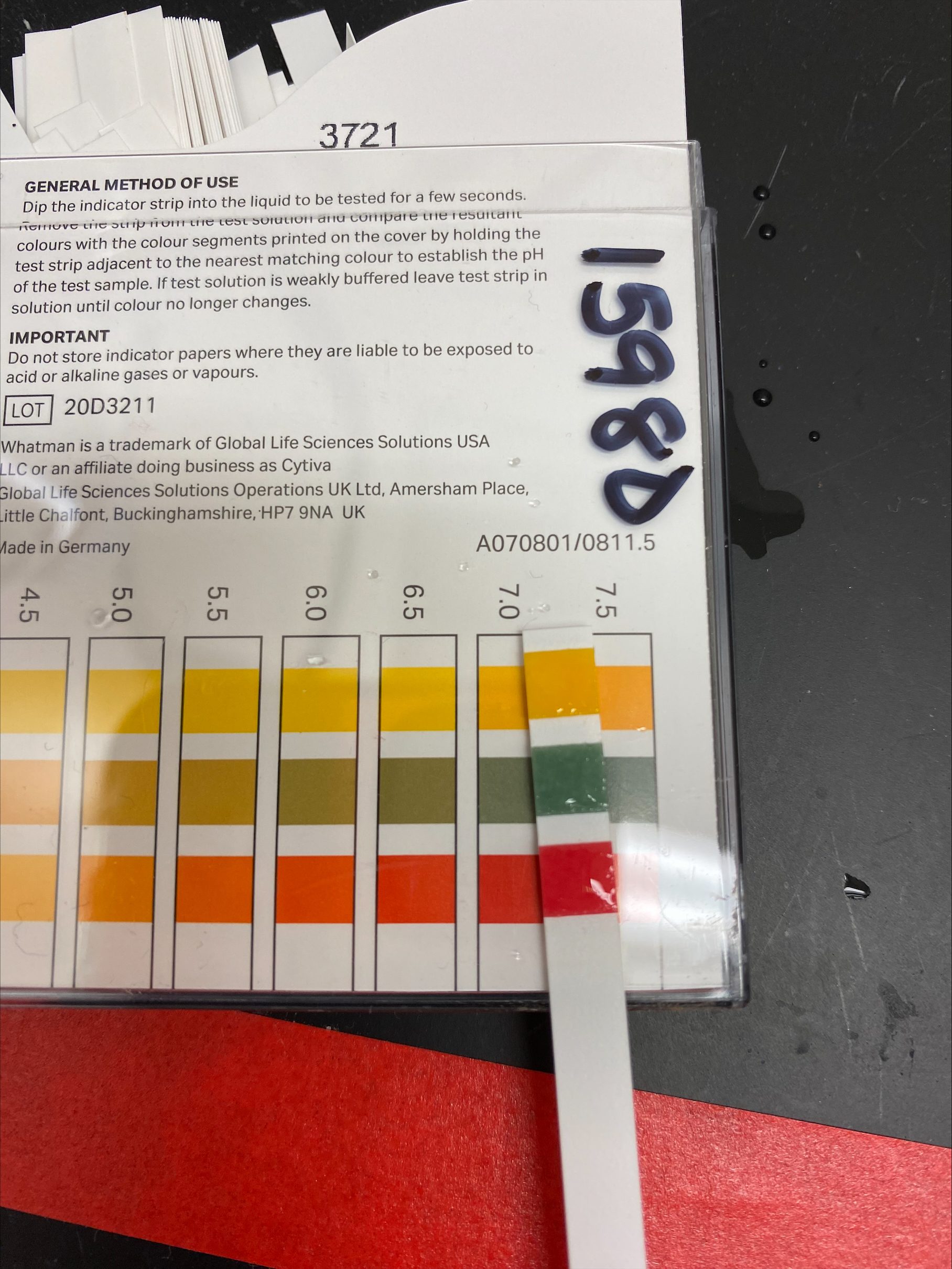
- water pH:
- I made 1N HCl by using our stock HCl that is 35-38%
- 8.3mL stock HCl
- 91.7mL Di-water
- I did all of this in the hood wearing a lab coat, and made sure to add the acid to the water and mixed
- Then I used a transfer pipette to drop in HCl to the ringers solution until the pH lowered to about 7, unfortunately I can’t completely tell how specific the pH is, and I might have gotten it a little under
- Added the remaining 50mL of DI-H20
- Mixed and vacuum filtered the solution through at 0.45um filter into a glass bottle
- Autoclaved the bottle on setting 3 and placed it in the cell culture fridge to store afterwards
20230216 Ovary Dissection and Primary Cell Culture
- Isolated out 50 females into empty vials, 5 flies per vial about an hour before starting so the would hopefully poop out much in their guts
- Females were age “4”
- Prepared:
- a small beaker with room temp ringers solution
- a dish with 70% ethanol
- a dish with DI-water
- Rob showed me how to do dissections, but all flies except 2 were done by me
- Used microscope slides and dissecting forceps
- Steps of each dissection:
- Put a drop of ringers on the microscope stage
- Placed a blank glass slide on the water drop
- Added a drop of ringers to one side of the slide
- Put one vial of flies to sleep on the CO2 pad
- Took a fly with forceps and dipped it into 70% ethanol, and then into the DI water
- Placed the fly on the droplet of ringers on the slide
- Use the forceps to try to pull off some tergets on the abdomen of the fly while holding the thorax with the other forcep
- Tried as best as possible to squeeze out the ovaries and not break the gut
- This was tough, many flies just fell apart as I dissected, or had small ovaries that I had to dig through the gut to get to
- Picked up the isolated ovaries and put time into the ringers solution beaker
- Dipped the forceps in 70% ethanol
- This was repeated for ~45 flies
- Then the beaker with the ovaries was taken to the cell culture hood in 4012
- There I had fly extract, mushroom extract, and mediums warmed to room temperature
- Put a mesh 100um cell strainer in an autoclaved flask and poured the ovaries in through the mesh. To get any stuck on the beaker I used embryo wash to wash them into the strainer
- Squirted 70% ethanol over the strained ovaries for about 1 minute to try to sterilize them
- Washed the ovaries out into a 50mL conical with 7mL of 10% FBS Schneider’s medium
- Put that liquid into a 10mL glass tube and let the ovaries settle (did not need to centrifuge)
- Removed all the liquid from the 10mL tube
- Washed the ovaries again with 7mL of 0% FBS Schneider’s medium
- Removed all the liquid from the 10mL tube
- Added 3mL of 10% FBS Schneider’s medium
- Transferred the liquid and ovaries to the 7mL dounce homogenizer
- Homogenized the ovaries as thoroughly as possible
- Moved the homogenate to a new T25 flask with 16mL of 10% Schneider’s medium
- Put 9mL of that liquid into a new T25 flask
- Added 1mL of fly extract to the flask with 9mL ovary homogenate
- Added 400ul of mushroom extract to the flask with 10mL ovary homogenate
- Placed both flasks in the 23C incubator