Testing Cell Transfection Reagents and pAc5 Plasmid on Primary innubila Cells
- I am using a 12 well plate of innubila primaries that I made on 9/28. There are wells with “supernatant” in them, these are wells with a lot of debris from the primary generation process and they also have some cells. I did not use these wells but they are on the plate
- Because I am limited on wells of cells, I am only going to use wells of just primary cells as the control. I will not get any wells that have transfection reagent only or plasmid only. Based off of the S2 transfection and cell counts from that I think this is fine
- Plate layout:
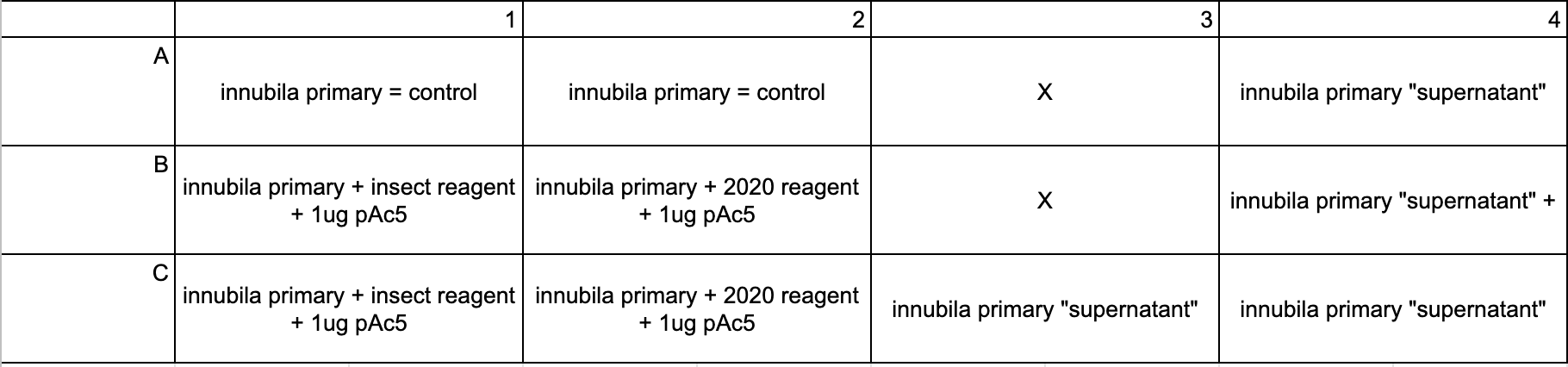
- For this I made only 2 master mix tubes
- Plasmid DNA is at ~1ug/ul
- All of this was done at room temperature in the cell culture hood. Transfection reagents were thawed at room temp, vortexed, and spun down before use
- Tube 1
- 200ul Schneider’s medium
- 2ul concentrated pAc5 plasmid
- 4ul insect reagent
- Tube 2
- 200ul Schneider’s medium
- 2ul concentrated pAc5 plasmid
- 6ul 2020 reagent
- Each tube was pipette mixed gently
- Tubes incubated in the hood for 30 minutes
- Then the reagents were dripped into the wells, mostly in the middle because most of the primary cells are clustered into the middle of the dishes:
- Added 103ul of tube 1 to wells B1 and B2
- Added 104ul of tube 2 to wells C1 and C2
- Incubated plates at 23 degrees for 24 hours then checked. The plate was always kept in the incubator between checks
20221020 Checking transfections
- No experimental wells showed any evidence of GFP after 24 or more hours
- I realized I should have used S2 cells as a positive control
- There was a lot of autofluorescence in the primary cells, and it was about the same amount for control and treatment. I think this is due to the amount of debris/clumps of cells with primary
- Some images from the plate are represented here