p47 and RPL11 PCRs and a HMW Gel on the Hirt/Phenol-chloroform Virus Extraction Samples
I got DNA out of at least the 1.2mL samples, but I need to know if there is virus DNA in there, and if there is fly cell DNA still in there too. I also need the virus DNA to be intact (HMW at ~150kb), so I ran an overnight gel to check that.
20220705 p47 and PRL11 PCRs
- p47
- I want to use the 0.2mL sample even though nothing showed up on the Qubit, there is still a chance there is DNA in there
- So I have 3 samples, plus 1 for a positive control, and plus 1 for a negative control
- Made master mix on ice:
- 5ul GoTaq * 5.5 = 27.5ul
- 0.25ul p47_F * 5.5 = 1.375ul
- 0.25ul p47_R * 5.5 = 1.375ul
- 3.5ul molecular grade water * 5.5 = 19.25ul
- Vortexed and spun down master mix
- Added 9ul master mix to 5 strip tubes
- Added 1ul of DNA sample to appropriate tubes
- Added 1ul of 3mL HMW extraction to the positive control tube
- Added 1ul of molecular grade water to the negative control tube
- Vortexed and spun down strip tubes
- Placed tubes in the p47 PCR program
- RPL11
- Using the same sample scheme as above
- Made master mix on ice:
- 5ul GoTaq * 5.5 = 27.5ul
- 0.25ul p47_F * 5.5 = 1.375ul
- 0.25ul p47_R * 5.5 = 1.375ul
- 3.5ul molecular grade water * 5.5 = 19.25ul
- Vortexed and spun down master mix
- Added 9ul master mix to 5 strip tubes
- Added 1ul of DNA sample to appropriate tubes
- Added 1ul of 3mL HMW extraction to the positive control tube
- Added 1ul of molecular grade water to the negative control tube
- Vortexed and spun down strip tubes
- Placed tubes in the RPL11 PCR program
- Ran a 2% gel for 35 minutes at 90 volts after the PCR programs were done:
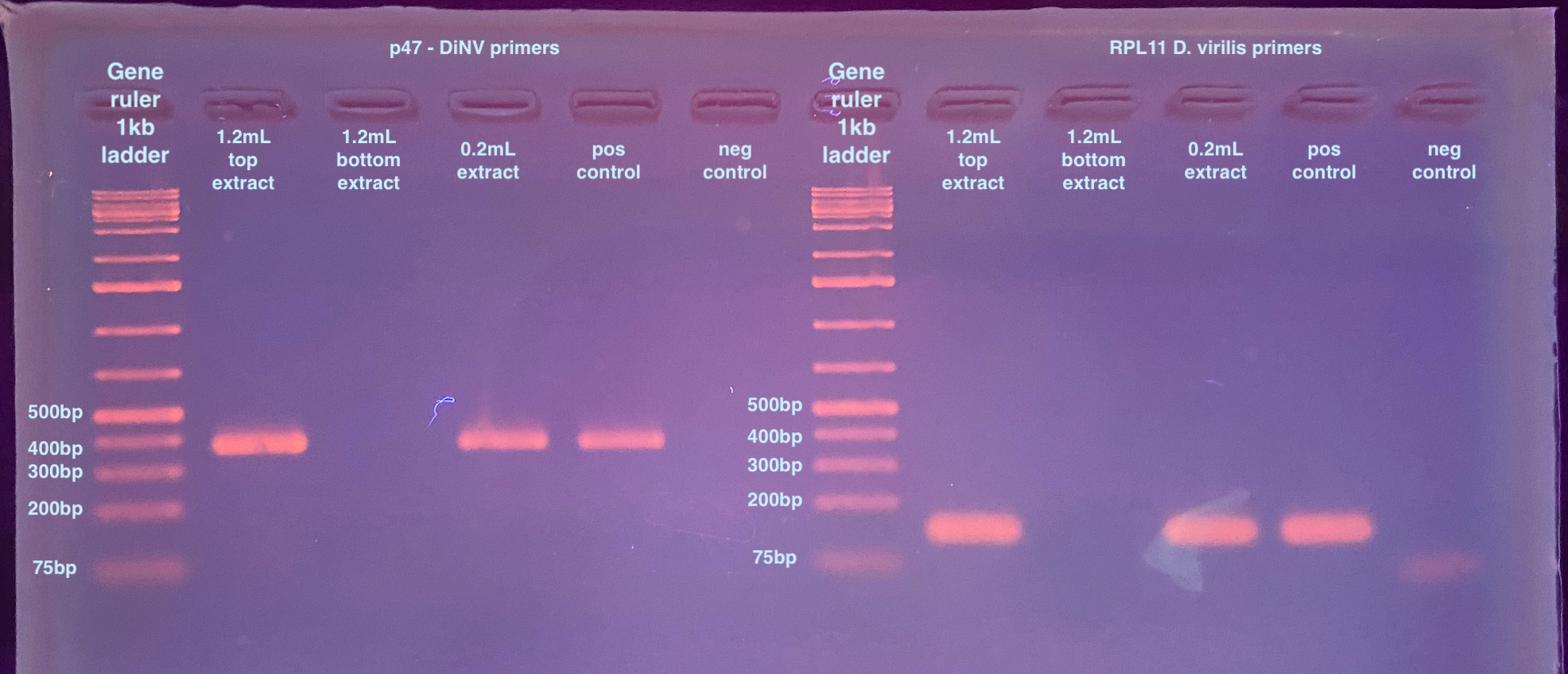
This tells me a couple of things. I definitely have virilis DNA contaminating my extraction. There was also DNA present in the 0.2mL sample. And the bottom of the 1.2mL extraction, even though it “had DNA” somehow the PCR didn’t work. Considering how bad the extraction went, I won’t consider this result final. I don’t want virilis DNA in my extraction however. So this is not a good sign. I want to try the extraction again (correctly!) and also use phase lock tubes to avoid getting the phases mixed again.
HMW gel 20220726
- Prepped 0.8% gel:
- 180mL of 1X TAE
- 1.4g agarose
- Microwaved on 1min increments for 4 minutes
- Saved 1mL of gel and put in the heat block at 65 degrees
- Let gel cool ~10 minutes before pouring
- Taped up the sides of the casting tray
- Poured the gel with the wide combs
- Let gel set for ~20 minutes
- Sliced 1 round of PFG marker into the first well
- Took gel tube out of the heat block and let sit ~3 minutes
- Filled the PFG marker well with gel to cap it
- Waited ~3 minutes for that gel to set
- Placed gel in the gel box and took off the tape
- Added 16ul of 48kb ladder to the next well
- Prepped samples:
- Added 15ul of sample to new strip tubes
- Added 3ul of 6X loading dye to each tube
- Flicked to mix and spun down
- Added the total volume of samples to the next wells
- Ran gel overnight at 40 volts for 16.5 hours
- Stained gel the next morning for ~30 minutes
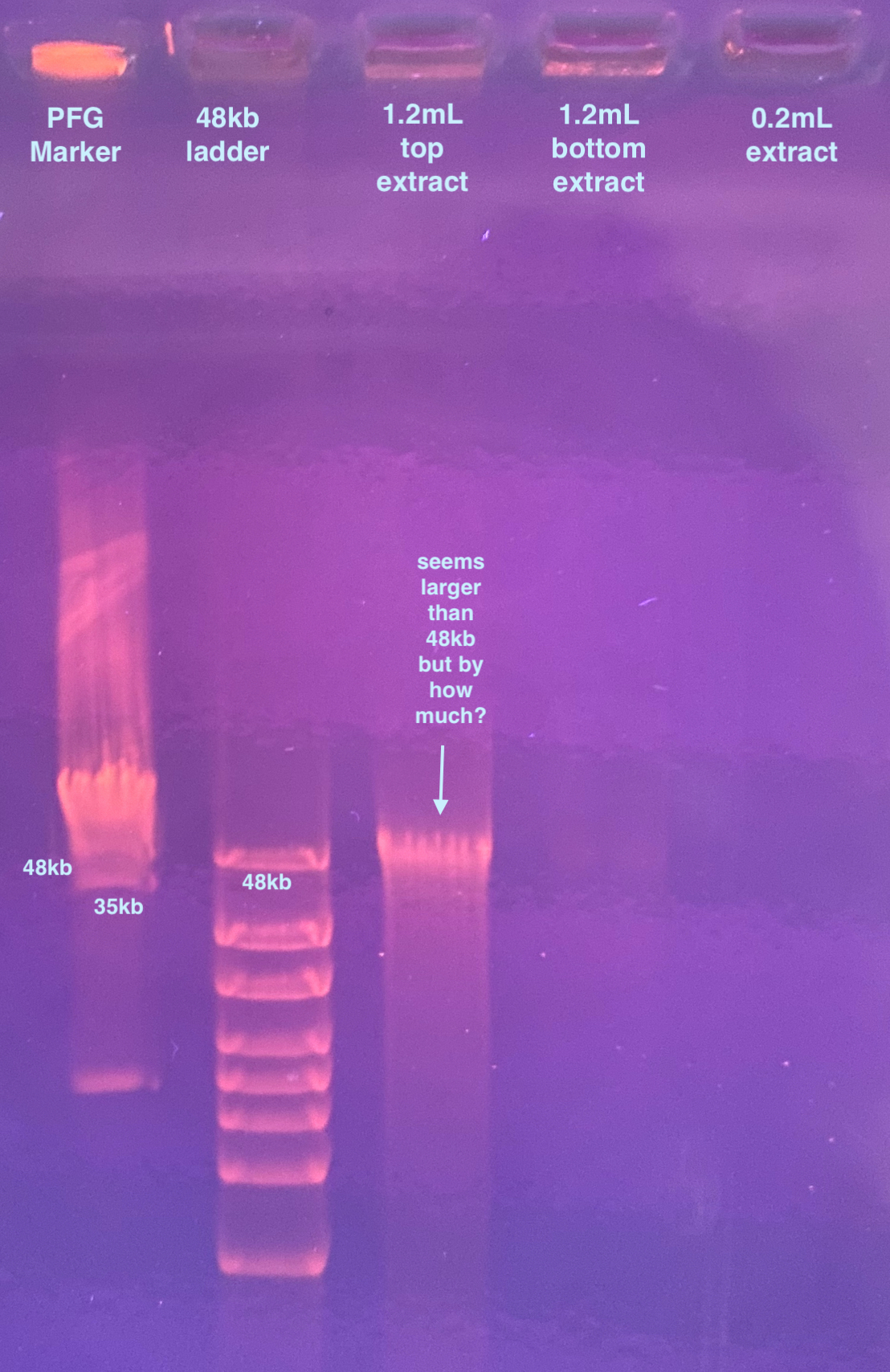
Really only the 1.2mL top sample shows up (others were probably too low concentration). It looks to me like the sample is above the 48kb marker, but by how much I’m not sure. I am worried that this means that the virus DNA is not quite intact. There is smearing of course but it seems minimal.