Testing 77kb Guide RNAs 86 and 92 on the 77-1 PCR Product in a Cas9 Test Digestion
Following this protocol from NEB.
Using 86 and 92 sgRNAs synthesized previously.
And 77-1 PCR product amplified here, cleaned with beads, and quantified.
Determining sgRNA concentration in nM
- Total RNA from synthesis and purification was:
- 86: 2.6ug
- 92: 678.6ng
- Length should be ~100nt
- Used NEB mass to mole converter to find the pmol of each:
- 86: 80.83pmol
- 92: 21.1pmol
- This needs to be converted into nM
- 1 pmol/ul = 1000nM
- Total volume sgRNA is in is ~39ul
- Use conversion equation:
- 86: 80.83pmol / 39ul = 2.073 pmol/ul * 1000 = 2073nM
- 92: 21.1pmol/39ul = 0.54pmol/ul * 1000 = 541nM
- I need sgRNAs at a 300nM concentration so this is good for both
Determining nM concentration of PCR product
- My 77-1 PCR product is 26.5ng/ul in 29ul
- The size is 1,414bp
- Equation for DNA ng/ul to nM:
- nM = ((ng/ul)/(660g/mol * size in bp)) * 1000000
- 77-1: ((26.5ng/ul)/(660g/mol * 1414) * 1000000) = 28.39nM
- I need my DNA at 30nM concentration, this is close enough
- I was not able to do the 77-2 PCR product today because it was at too low a concentration
Dilutions
- I do not need to dilute my PCR product because it is already just about at the right concentration
- I need to dilute my sgRNAs to 300nM, and I’m going to make 30ul of it
- 300nM * 30ul = 9000nmols needed
- 86: 9000nmols/2072nM = 4.34ul sgRNA 86
- 92: 9000nmols/541nM = 16.6ul sgRNA 92
- Need to bring total volume up to 30ul with molecular grade water
- 86: 30 - 4.34 = 25.66ul
- 92: 30 - 16.6 = 13.4ul
- Dilutions of sgRNA was done on ice
- Cas9 needs to be diluted to uM, the stock is 20uM
- 1ul Cas9
- 19ul dilutent B
- Dilution of Cas9 was also done on ice
Set up and Procedure
| tube number | PCR product | sgRNA | Cas9 | reaction type |
|---|---|---|---|---|
| 1 | 77-1 | +sgRNA 86 | No Cas9 | control |
| 2 | 77-1 | No sgNRA | + Cas9 | control |
| 3 | 77-1 | No sgRNA | No Cas9 | control |
| 4 | 77-1 | +sgRNA 92 | No Cas9 | control |
| 5 | 77-1 | +sgRNA 86 | + Cas9 | digest test |
| 6 | 77-1 | +sgRNA 92 | + Cas9 | digest test |
- Set the thermocycler program:
- 25 decree C hold
- 25 degree C 10 minutes
- 37 degree C hold
- 37 degree C 15 minutes
- Thawed all reagents on ice and made mixes on ice
- Made master mix:
- 20ul molec grade water * 7 = 140ul
- 3ul NEB buffer r3.1 * 7 = 21ul
- Pipette mixed and spun down
- Added 23ul master mix to each tube (of 6 strip tubes)
- Added 3ul 300nM sgRNA 86 to tubes 1 and 5
- Added 3ul 300nM sgRNA 92 to tubes 4 and 6
- Added 3ul water to tubes 2 and 3
- Added 1ul 1nM Cas9 to tubes 2, 5, and 6
- Added 1ul water to tubes 1,3, and 4
- Pipette mixed tubes and spun them down
- Incubated the tubes in the thermocycler for 10 minutes at 25 degrees C
- Took out the tubes and added 3ul of 77-1 PCR to every tube
- Pipette mixed and spun down tubes
- Incubated tubes in the thermocycler for 15 minutes at 37 degrees C
- Took tubes out and added 1ul of Qiagen proteinase K to each
- Pipette mixed and spun down tubes
- Incubated the tubes at room temp for 10 minutes
Gel(s)
- I immediately ran a 1% gel for 30 minutes at 90V
- I used 15ul (half) of each sample and mixed that with 3ul of loading dye
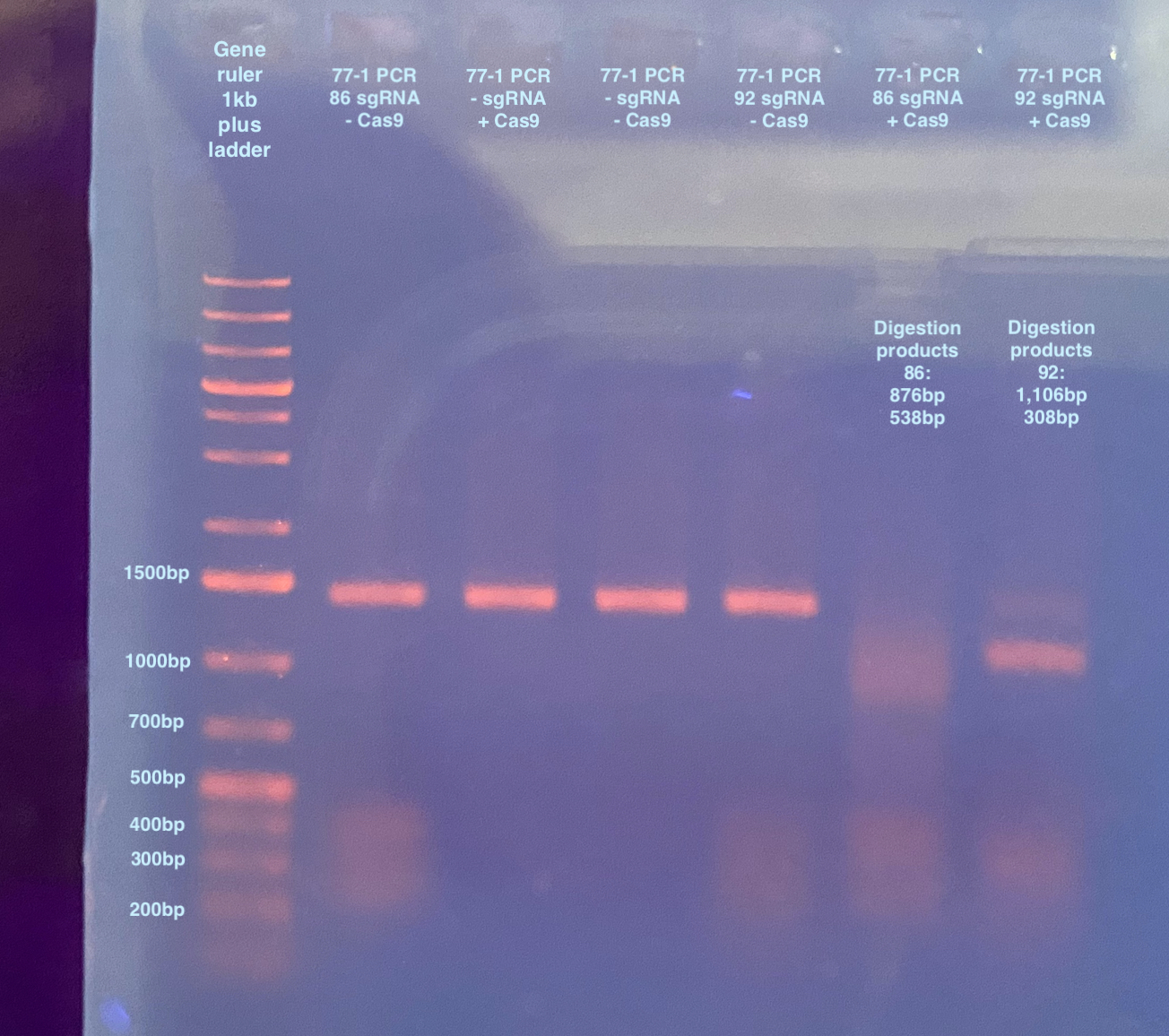
This image is not so good, but it seems like the digestion worked? The smearing in the wells that has sgRNA are weird to me. I decided to run the gel again with a higher percentage, and also ad in a well with pure sgRNA:
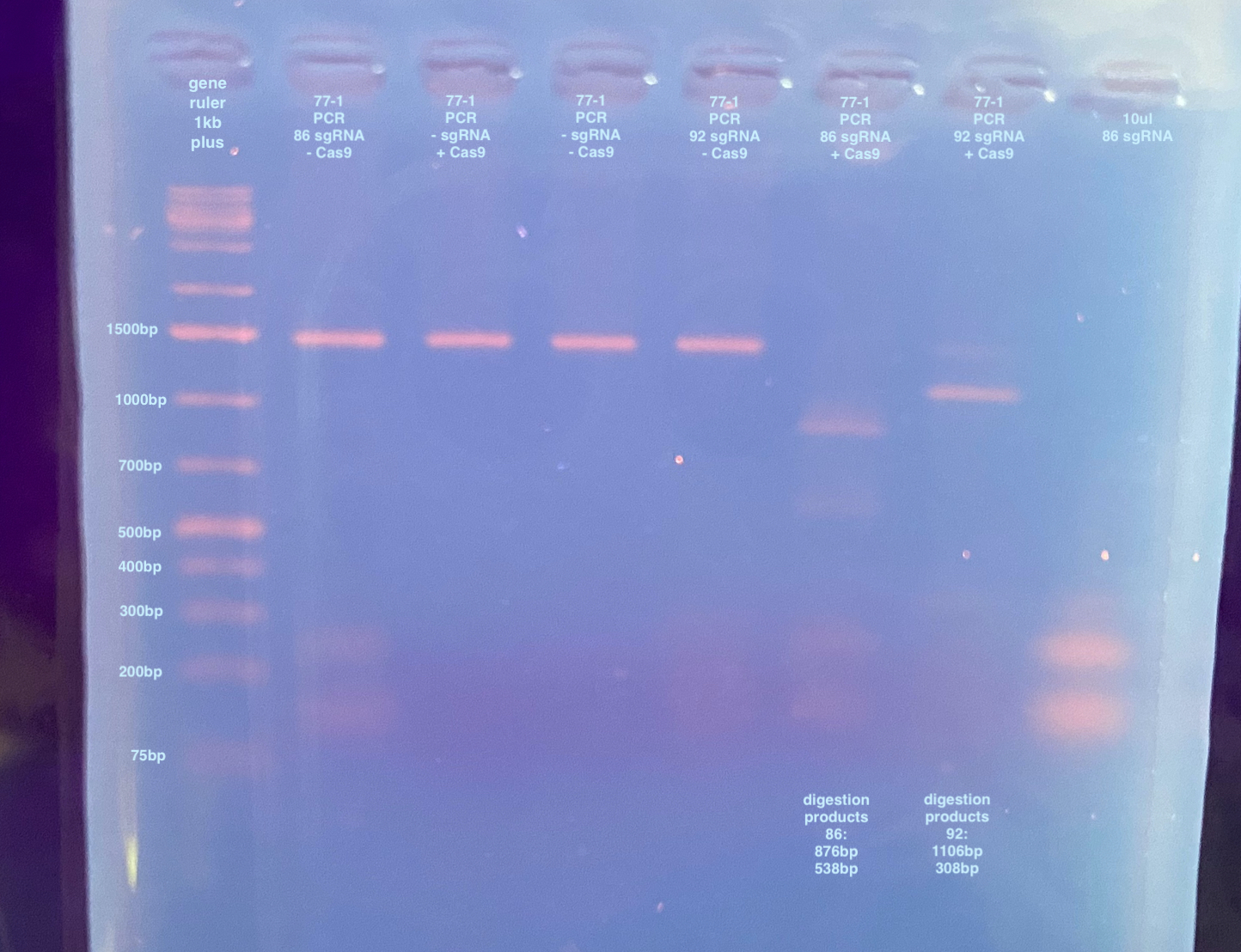
This was slightly clearer but I couldn’t get a good image. I also “enhanced” the image with more contrast to try to see the separate bands.
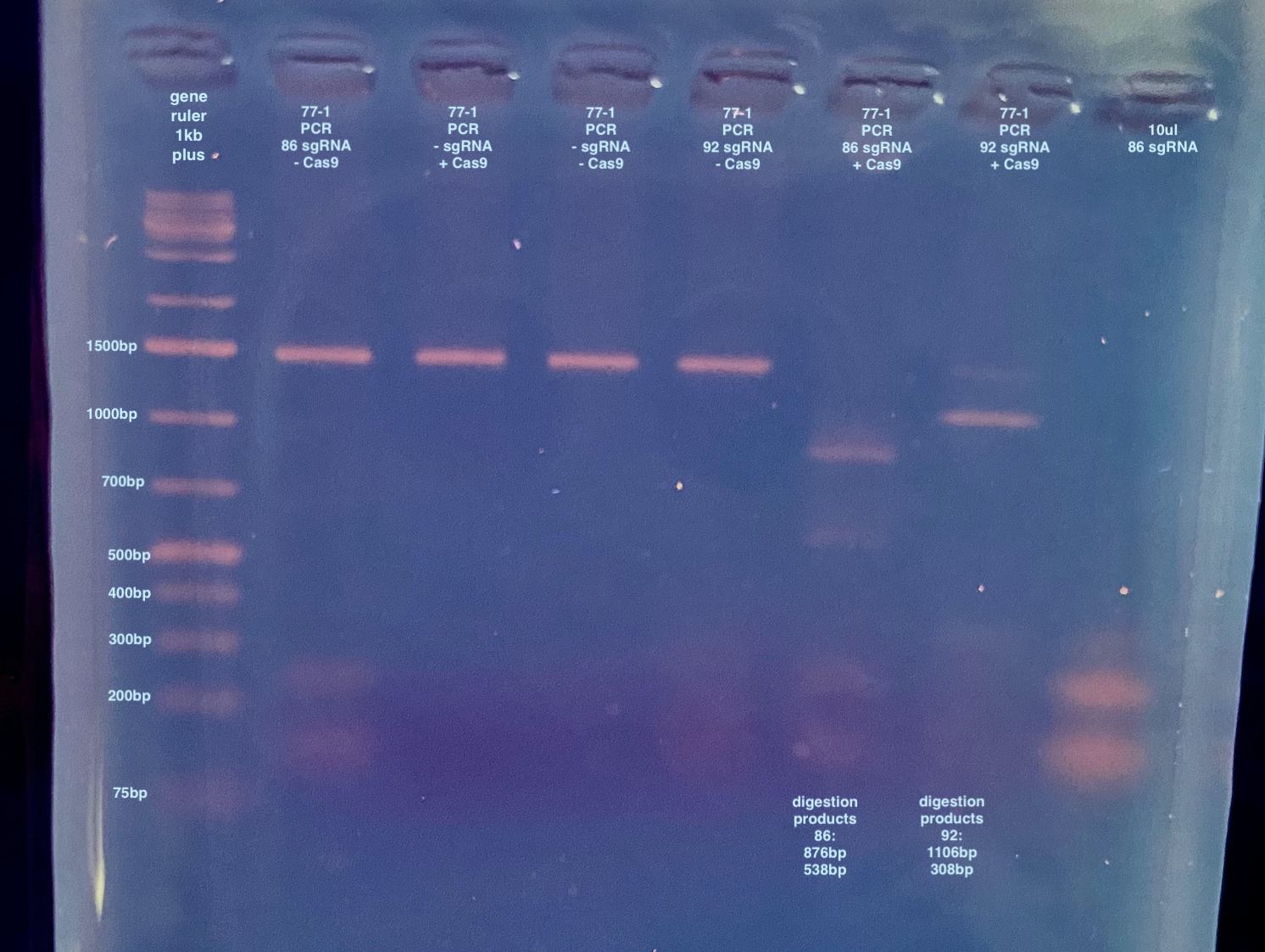
I feel like I can faintly see the correct digestion products in the second to last 2 wells. Although it looks like sgRNA 92 doesn’t cut completely. I am confused though why the sgRNA on it’s own has 2 bands. The NEB synthesis kit instructions say it’s supposed to produce 1 product ~100nt long…. I mean I guess it works anyways??
I stll want to check these sgRNAs on the 77-2 PCR product.