Using 4 Different Laying Plate Types to See What D. innubila Lays on Best
20220110 Made Fresh Apple Juice Plates and Mushroom Agar Plates
- Made new apple juice agar plates, and poured them into the small petri dishes as well as the large ones
- Also made mushroom agar (Lab recipe, but used frozen mushrooms that were then autoclaved) and poured into small petri dishes
- Made fresh yeast paste and autoclaved twice
20220111 Added Yeast to Plates
- Trying plain plates for both mushroom and apple juice, and also plates with a little yeast on them
- Experimental plates:

20220112 Setting up Flies
- Used the 8 vials of flies that had been saved from the week before’s innubila cell culture attempt
- Used 2 vials per cage, and had 4 cages set up at a time. One mushroom plate, one AJ plate, one mushroom plate with yeast, and one AJ plate with yeast
- Did not knock out flies when adding to the cages so some flies were lost but happiness of flies should have remained
- Noted that the mushroom plates were kind of wet
20220113 Cell Culture Day 1
- Had Rob show me how to pick out eggs from the plates with a tooth pick
- Transferred flies to new plates to lay again
- Many of the flies on the mushroom plates had gotten stuck and died, mostly because the plates were too wet
- Imaged each plate to count eggs x
- Picked out eggs and put them on a yellow 100um filter
- Returned to 4012 and mostly followed the Prepping Cell Culture Protocol with some modifications:
- Used 10% FBS Schneider’s medium without gentamicin or 0% FBS Schneider’s medium without gentamicin made fresh that day
- Did not use trypsin to minimize the number of washes needed to prevent loss of cells/eggs
- Instead, I used 0% FBS medium to wash out the beach solution, 3 washes, then homogenized the eggs in 2mL of 10% medium
- There was no waiting period after homogenization, and the homogenate was added to a flask with 3mL of 10% medium and put into the incubator at 23 degrees
20220114 Cell Culture Day 2
- Transferred flies to food vials
- Imaged plates to count eggs x
- There were a lot more eggs on the mushrooom plates today, but the darkness/opaqueness of the plates made it very hard to see in the images
- Picked out eggs and put them in a beaker with some eggs wash buffer
- Returned to 4012 and mostly followed the Prepping Cell Culture Protocol using the same modifications from above
- Tried to remove as much of the agar pieces or at least break them up by swishing a paintbrush on the filter while washing it with egg wash


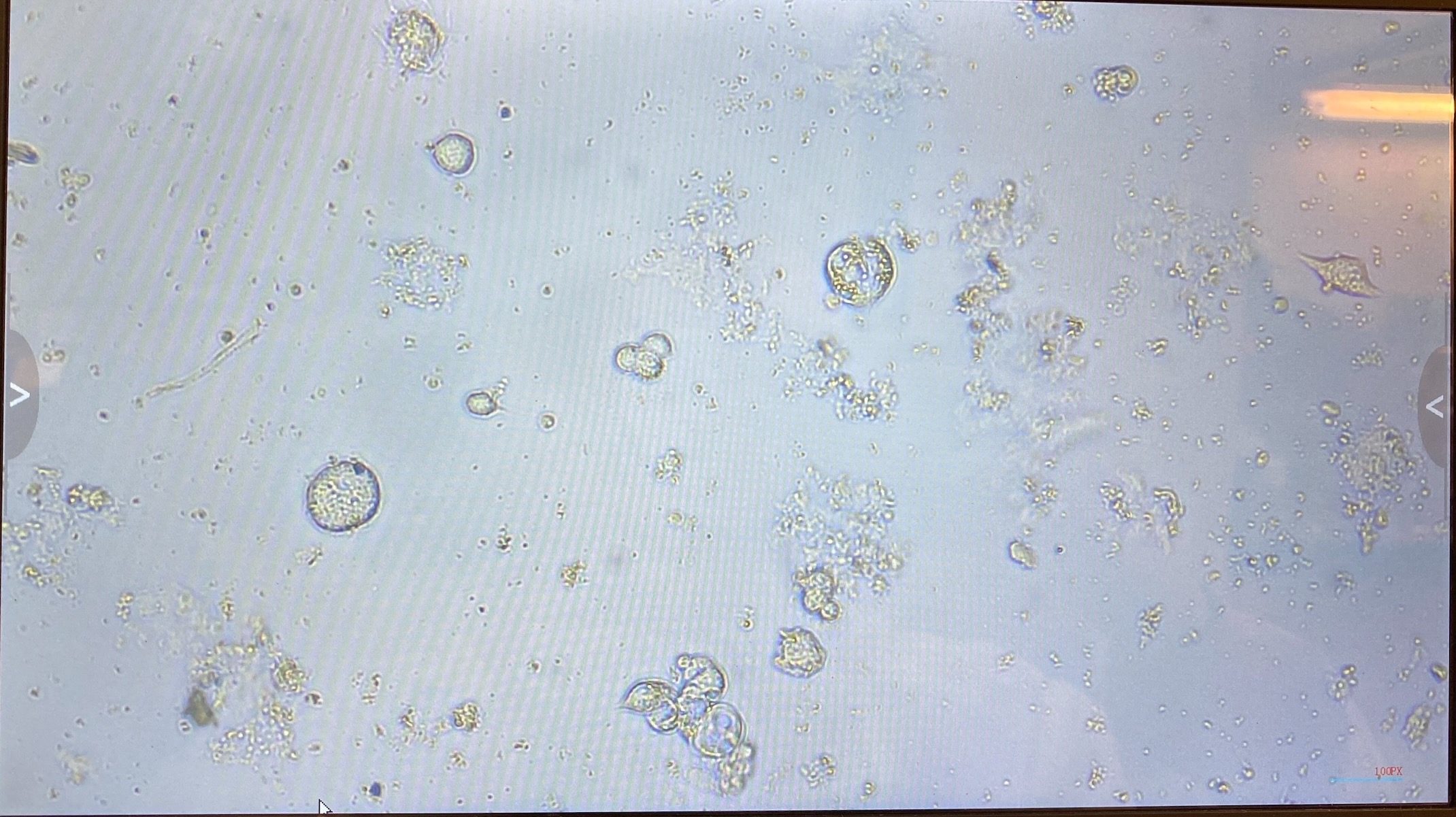
- Imaged flasks from both days. There was a lot of debris but there was also visible cells and cell clumps!
- 20220113 flask:
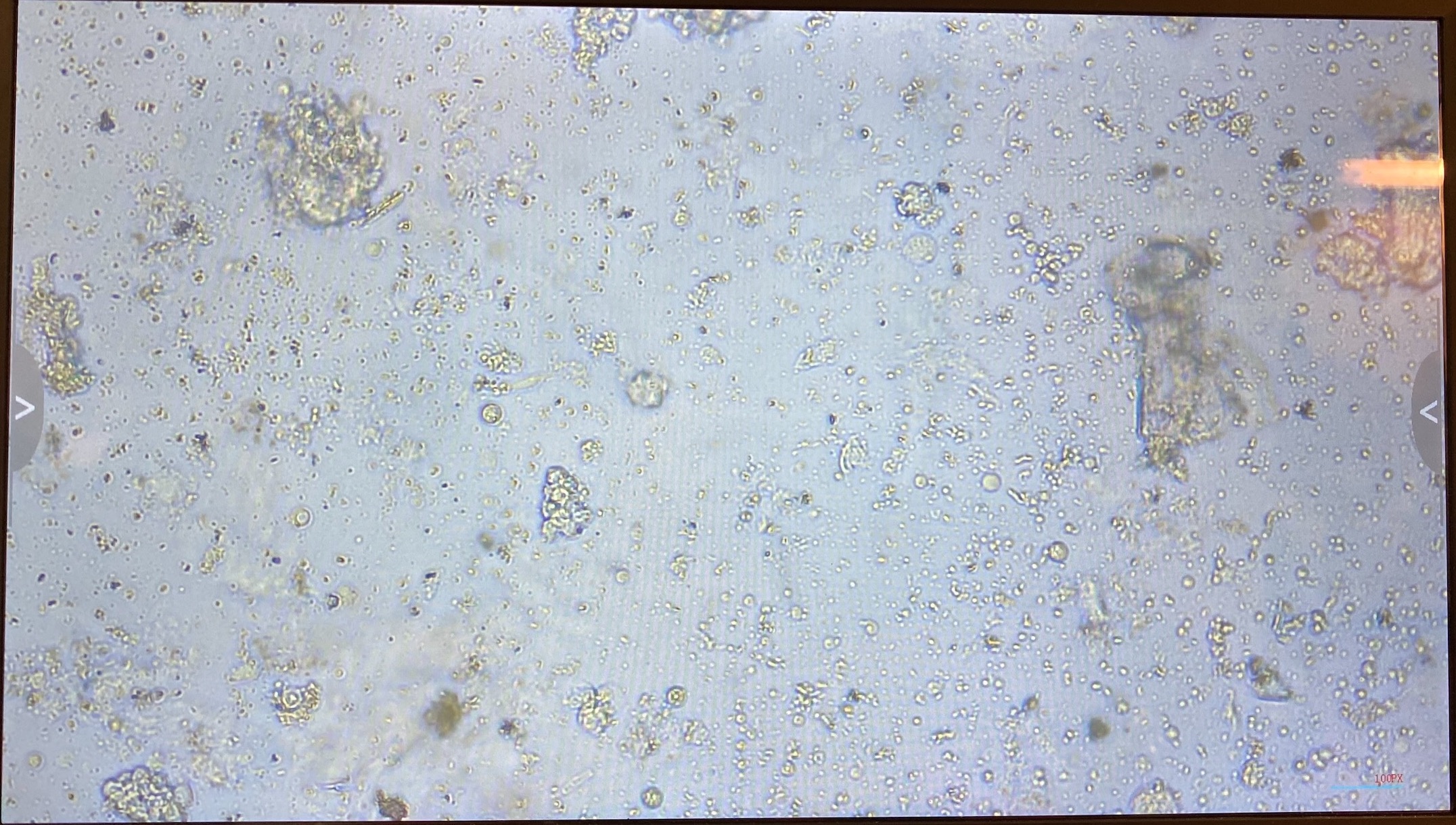
- 20220114 flask:
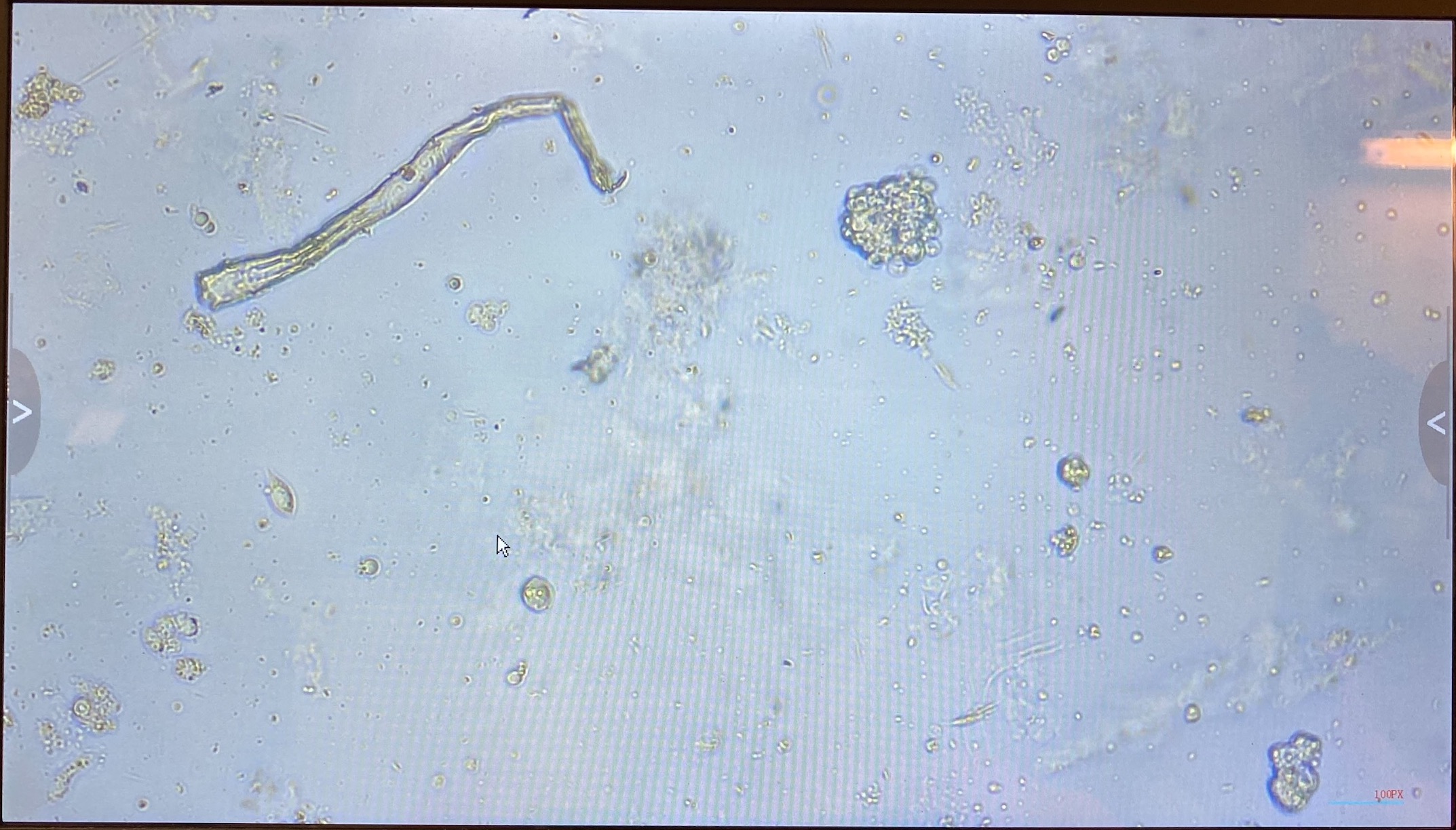
20220121 Imaging Flasks Again and Fluid Addition
- Added 1mL of 20% Schneider’s medium to the 20220114 flask
- Imaged flasks, there is still debris of course, but the cells look big and still alive, some are even contracting