Plating pBeloBAC11 and pBACe3.6 from Agar Stabs, Growing Liquid Cultures, and Making Glycerol Stock
20220105 Agar Stab Plating
- Both BACs have chloramphenicol antibiotic resistance. pBACe3.6 should be grown at 12.5ug/mL and pBeloBAC11 should be grown at 15-20ug/mL. Because they’re so similar, they were both grown at 15ug/mL.
- Our stock chloramphenicol is at 50mg/mL in ethanol
- The LB plates are 25mL in volume
- The total amount of chloramphenicol needed per plate is 15ug/mL * 25mL = 375ug
- Calculation is : vol needed = amount needed in ug/concentration in ug of stock * vol factor of stock
- 50mg/mL is also 50ug/ul
- 375ug/50ug * 1ul = 7.5ul of chloramphenicol needed
- Because you don’t want to spread less that 10ul on the plate (too little volume) a little dilution was made: 15ul 50mg/mL chloramphenicol and 15ul
- Warmed up two LB plates in the 37 degree C incubator and labeled appropriately
- Made spreaders from pasture pipettes: melted two edges of the pipette in the flame of the bunsen burner to make a flat spreader. Kept the spreader on a kim wipe, the spreader edge not touching anything
- Kept the bunsen burner on continuously
- Added 15ul of the diluted chloramphenicol to the center of one plate and immediately spread it around the plate with the sterile spreader, rotating the plate continuously. Repeated for the second plate
- Used a plastic sterile loop to scoop out a small chunk from the agar stab an spread it across the appropriate plate, trying the break up the agar piece on the plate to spread around. Repeated for the second BAC/plate
- Placed plates agar side up in the 37 degree incubator overnight
20220106 Setting up Overnight Liquid Cultures
- Plates grew well overnight:
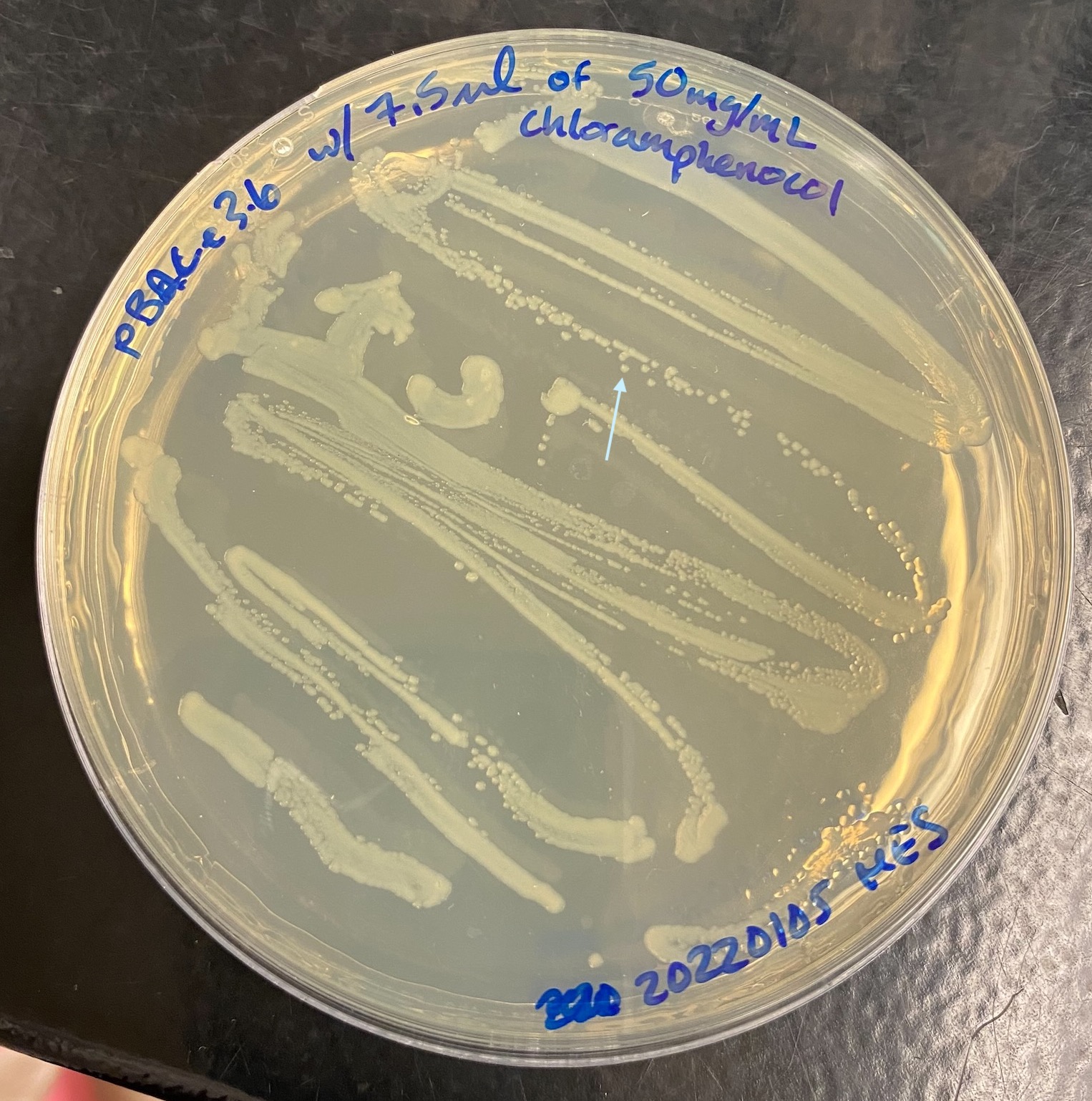
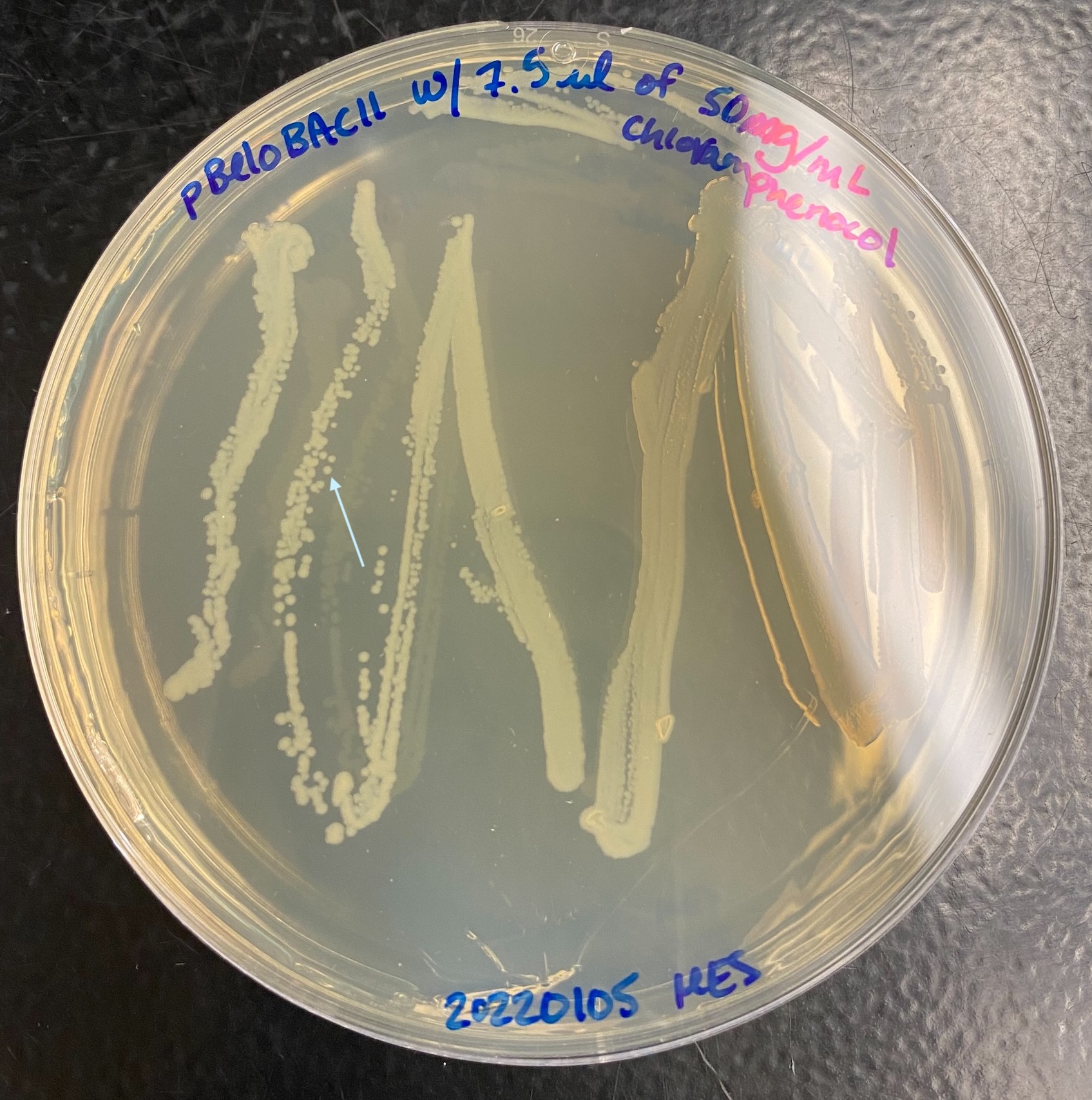
- LB test tubes have ~2mL in them
- Calculated the amount of chloramphenicol is needed:
- 30ug/50ug * 1ul = 0.6ul of chloramphenicol needed
- Turned on bunsen burner
- Added 0.6ul 50mg/mL chloramphenicol to 3 LB test tubes per BAC (2 are needed for the glycerol stocks, and 1 is a backup)
- Took a p2 pipette tip and picked a single colony from the plate and quickly dropped the tip into one of the test tubs. Hold the open plate facing the flame and pick the colony from behind. Single colonies are like the ones with arrows from the images
- Repeated picking colonies for the other 5 tubes
- Placed the test tubes in the 37 degree shaking incubator over night
- Parafilmed the plates and put them in the fridge for storage
20220107 Making Glycerol Stocks
- Test tubes were nice and cloudy
- 2 cryotubes were labeled for each BAC (one is a backup)
- 750ul of 50% glycerol was added to each cyrotube
- 750ul of liquid culture was added to their respective tubes and mixed
- Culture tubes were bleached
- Cryotubes were put in the glycerol stocks and backup boxes in the -80 freezer
- Added these to the bacterial spreadsheet on the google drive: 134 and 135