Cell Culture Preparation of D. innubila Genome Eggs
20220104 Making Yeast Plates
- Used yeast paste made by Kent, potentially the week of 20211220
- Then that afternoon, made 2 apple juice yeast plates that were completely covered in a layer of yeast
20220105 Setting up innubila Flies to Lay
- Set up 4 vials of D. innubila flies from my stock, and 5 vials from Rob’s stock to lay on the yeast plate at about 2pm
20220106 Day 1 Cell Culture
- Collected the apple juice plate in the afternoon and noticed a lot of dead flies. I added 3 more vials of flies to the cage and set it up again with a new yeast plate
- Followed the Prepping Cell Culture Protocol for filtering the eggs
- Used 20% serum Schneider’s medium made on 20211216
- Looked at the yeast plate, and didn’t see any eggs. After filtering, there were no eggs visible (confirmed with Rob)

- Cell culture stopped and I did not proceed
20220107 Day 2 Cell Culture
- Collected the apple juice plate at ~10am and saved the flies
- Followed a modified Prepping Cell Culture Protocol - see adjusted steps below
- Used the 0% FBS and 20% FBS Schneider’s medium made on 20211216
- There were a few eggs from this plate, it was very hard to see:
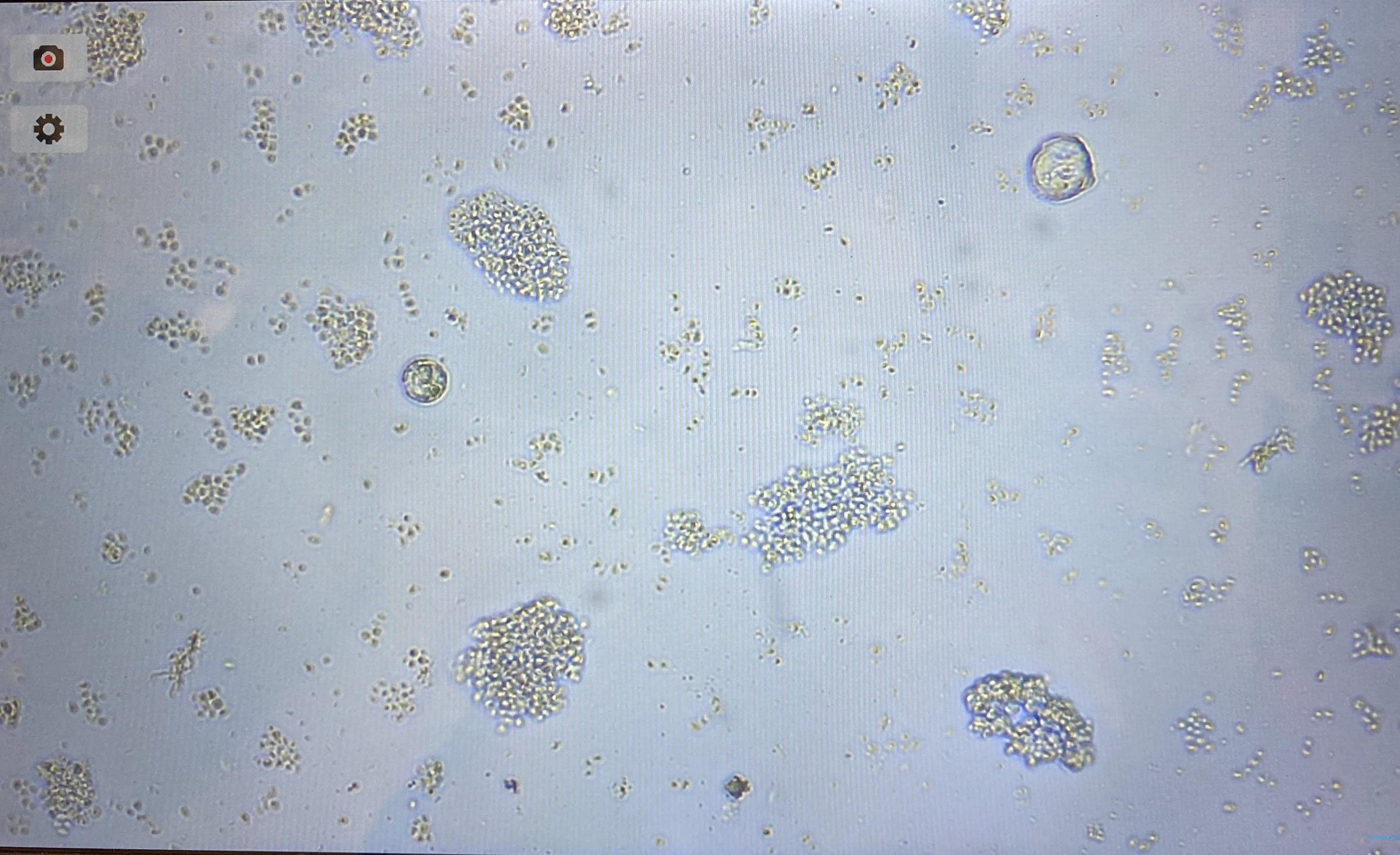
- After filtering, there were about 30-40 eggs:

- I modified the cell culture preparation slightly to remove trypsin, meaning less washes to try to keep as many eggs/cells as possible
- After the 50% beach soak, the eggs were washed 3 times with 0% FBS medium
- Then they were homogenized in 2mL of 20% FBS medium and immediately transferred to 3mL of 20% FBS medium in a single
- Flasks were looked at but not imaged until the next week
20220111 Imaging Flasks
- There are very few cells visible in this flask. A lot of debris is present, likely yeast. There was a lot of presumably yeast left in the filter, even after minutes of washing with egg wash and the squirt bottle