Testing Diluted DNA 10ul Reaction Volume, 20ul Reaction Volume, and Diluted DNA in 20ul Reaction Volume for “3mLB” Gel Extractions on p47 PCR (for DiNV detection)
20211220 20ul Reaction PCRs
- Made up a master mix for all the 20ul reaction volume reactions:
- 2ul NEB buffer * 15.5 = 31ul
- 2ul dNTPs * 15.5 = 31ul
- 2ul MgCL2 * 15.5 = 31ul
- 0.5ul p47_F * 15.5 = 7.75ul
- 0.5ul p47_R * 15.5 = 7.375ul
- 0.2ul NEB Taq * 15.5 = 3.1ul
- 9.9ul water * 15.5 = 151.9ul
- Kept master mix on ice
- Set up 13 strip tubes for the PCRs, tried using samples from the 1st attempt Zymo gel extraction, and the second attempt Zymo gel extraction (B)
- Added the appropriate amount of master mix, sample, and water to each tube:
| tube # | sample | ul master mix | ul DNA | ul water |
|---|---|---|---|---|
| 1 | 3mL-150 | 17 | 3 | 0 |
| 2 | 3mL-UP | 17 | 3 | 0 |
| 3 | 3mL-LOW | 17 | 3 | 0 |
| 4 | 3mLB-150 | 17 | 1 | 2 |
| 5 | 3mLB-LOW | 17 | 2 | 1 |
| 6 | 3mL-150-D | 17 | 3 | 0 |
| 7 | 3mL-UP-D | 17 | 3 | 0 |
| 8 | 3mL-LOW-D | 17 | 3 | 0 |
| 9 | 3mLB-150-D | 17 | 1 | 2 |
| 10 | 3mLB-UP-D | 17 | 3 | 0 |
| 11 | 3mLB-LOW-D | 17 | 2 | 1 |
| 12 | neg control | 17 | 0 | 3 |
| 13 | pos control | 17 | 1 | 2 |
- Vortexed and spun down and put in the PCR program for p47
- 95 degrees C 5 minutes
- 95 degrees C 30 seconds
- 55 degrees C 30 seconds
- 68 degrees C 30 seconds
- 68 degrees C 5 minutes
- Hold at 12 degrees C
- bold text is cycled through 30 times
- After, tubes were put in the fridge for overnight until a gel could be run
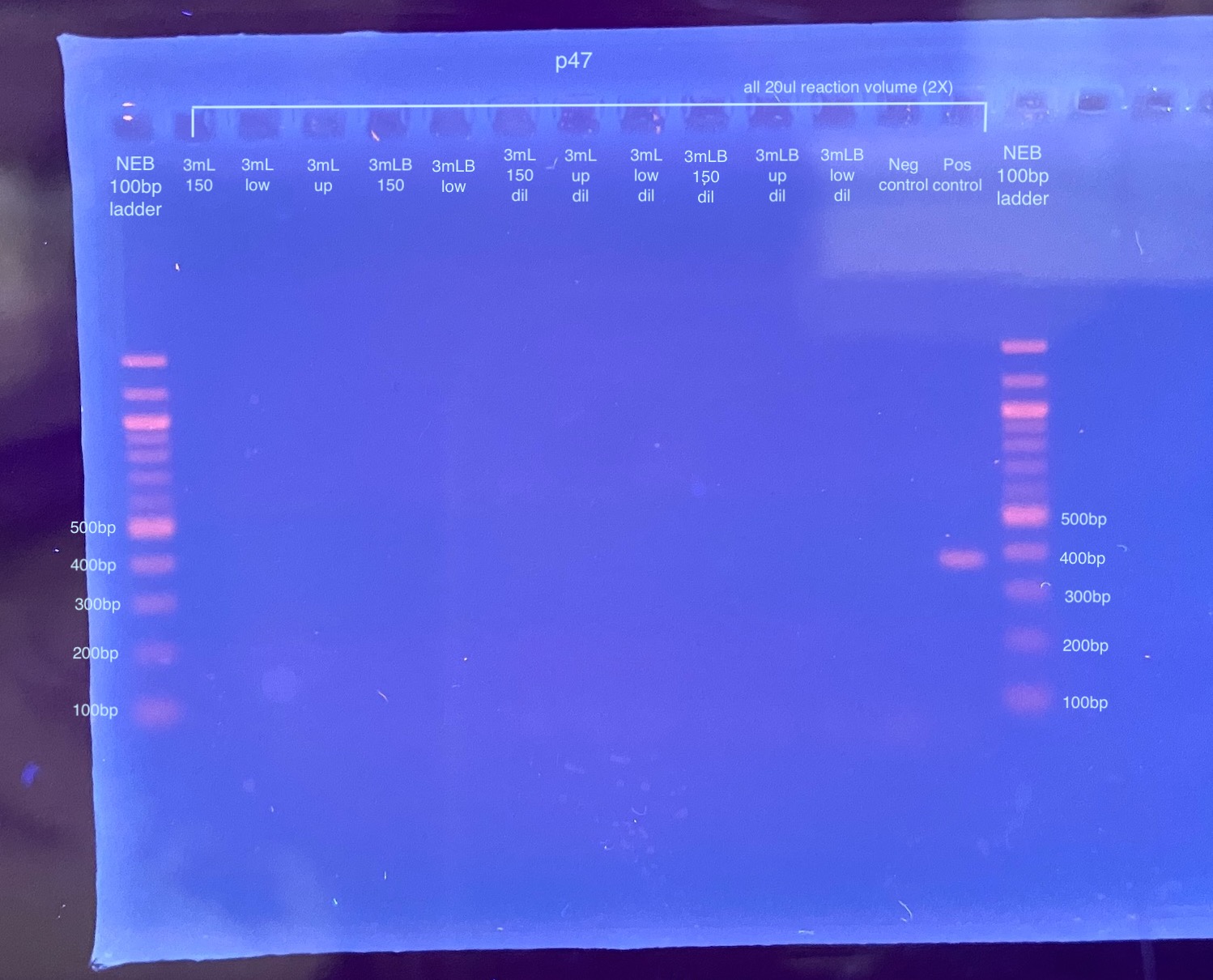
20211221 Gel of 20ul Reactions
- Ran all samples on a 2% gel for 30 minutes at 90V
- All samples except the positive control had no amplification…
20220103 Diluted DNA 10ul Reaction PCR
- Made up a master mix for 10ul reactions, only using the “B” diluted samples, and using the 3mL HMW DNA as a second positive control
- 1ul NEB buffer * 6.6 = 6.6ul
- 1ul dNTPs * 6.6 = 6.6ul
- 1ul MgCL2 * 6.6 = 6.6ul
- 0.25ul p47_F * 6.6 = 1.65ul
- 0.25 p47_R * 6.6 = 1.65ul
- 0.1ul NEB Taq * 6.6 = 0.66ul (not this was Taq that had been left out at some point)
- 4.4ul water * 6.6 = 29.04ul
- Kept master mix on ice
- Added mix, DNA, and water to strip tubes:
| tube # | sample | ul master mix | ul DNA | ul water |
|---|---|---|---|---|
| 1 | 3mLB-150-D | 8 | 1 | 1 |
| 2 | 3mLB-UP-D | 8 | 2 | 0 |
| 3 | 3mLB-LOW-D | 8 | 2 | 0 |
| 4 | 3mL HMW | 8 | 1 | 1 |
| 5 | neg control | 8 | 0 | 2 |
| 6 | pos control | 8 | 1 | 1 |
- Vortexed and spun down and put in the PCR program for p47
- 95 degrees C 5 minutes
- 95 degrees C 30 seconds
- 55 degrees C 30 seconds
- 68 degrees C 30 seconds
- 68 degrees C 5 minutes
- Hold at 12 degrees C
- bold text is cycled through 30 times
- A 2% gel was made the same day, and the samples were run for 30 minutes at 90V, again: the only amplifications are the two positive cintriks
