Cell Culture Preparation of 14091 Myd88 Eggs
2021213 Making Fresh Yeast Paste
- I made fresh yeast paste by using ~20mL of dry yeast, ~20mL of molecular grade water, and a few mL of apple juice. I added in some extra yeast to get the consistency right. Wanted it to be kind of like royal icing
- Autoclaved on program 3 twice on 20211213
2021124 Making Yeast Plates
- Made sure the scooper/spatula was autoclaved
- In the afternoon, made 2 apple juice yeast plates with 2 streaks of yeast on each plate
20211215 Setting up 14091 Myd88 Flies to Lay
- Set up 5 vials of 14091 Myd88 flies to lay on the yeast plate at about 2pm
20211216 Day 1 Cell Culture
- Collected the apple juice plate at ~9:45am and replaced the cage with a new plate for more laying
- Followed the Prepping Cell Culture Protocol
- Used freshly made that day 20% FBS Schneider’s medium, and 0% FBS Schneider’s medium:
- 2 50mL conicals No FBS:
- 49.5mL Schneider’s Drosophila medium
- 500ul 100X antibiotics
- 50ul gentamicin
- 1 50mL conical 20% FBS:
- 39.5mL Schneider’s Dosophila medium
- 10mL FBS
- 500ul 100X antibiotics
- 50mL gentamicin
- There looked like there were some eggs on the plate
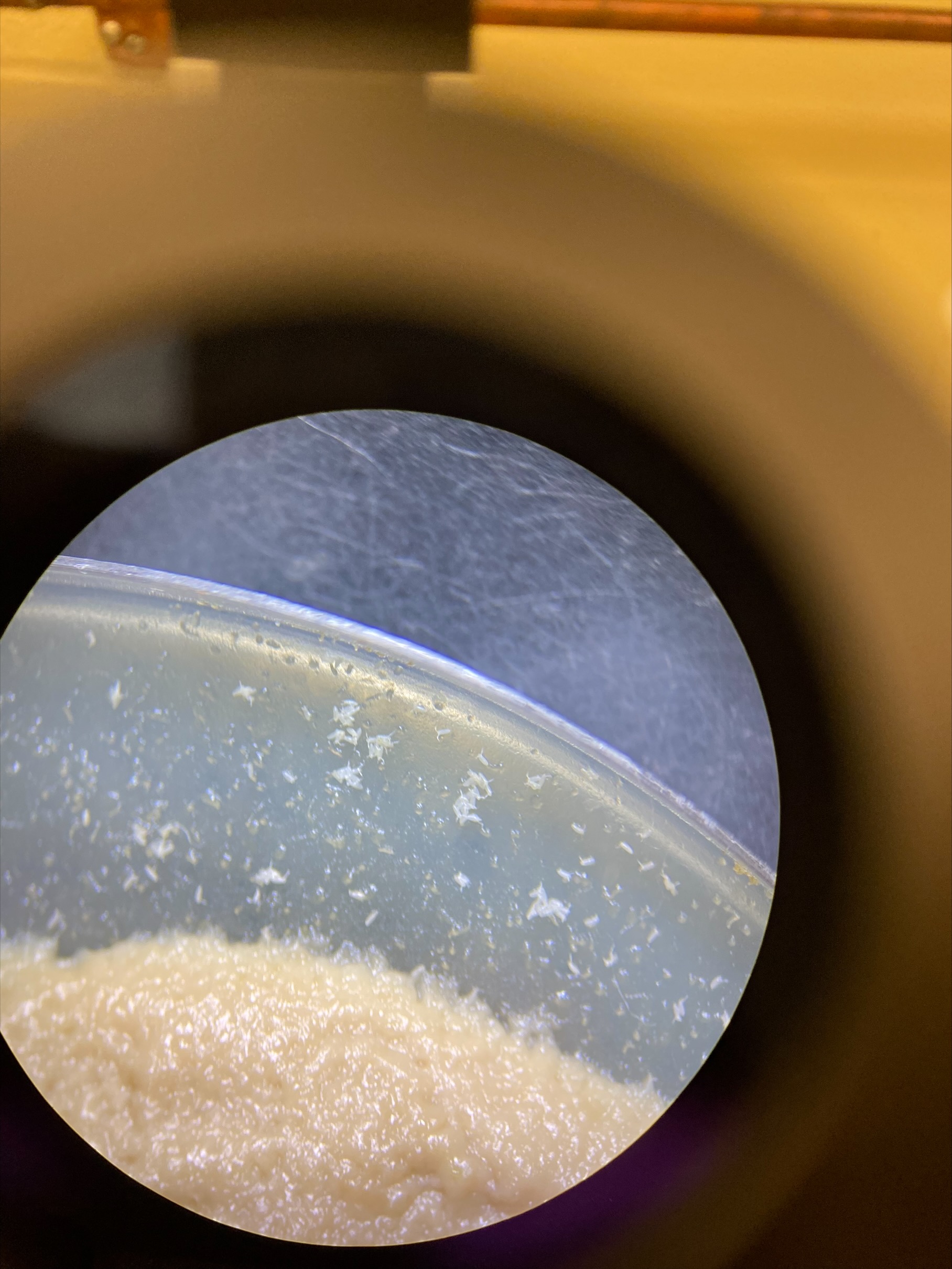
- Used a 100um filter to strain out the eggs, this seemed to keep a lot of yeast debris (brown specks in the image)

- When looking in the vial, it’s hard to tell how much is debris and what is eggs because the 50% bleach solution turns everything white

- The cell pellet ended up being pretty small
- Flasks were left to settle overnight and imaged the next day
20211217 Day 2 Cell Culture
- Collected the apple juice plate at ~9:45am and disposed of the flies
- Followed the Prepping Cell Culture Protocol
- Used the 0% FBS and 20% FBS Schneider’s medium made on the 16th
- There were many more eggs on the plate this day


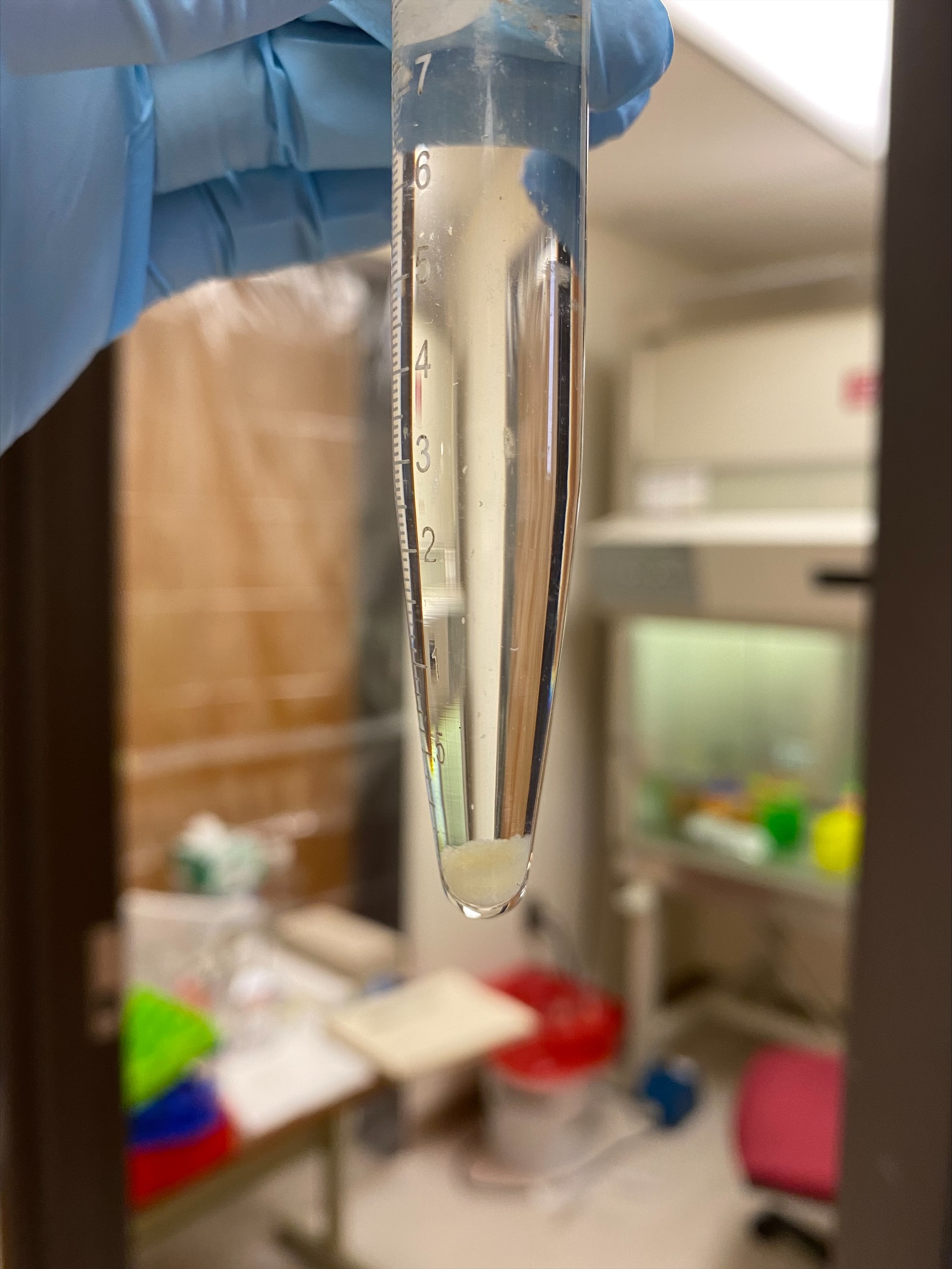
- I used the 70um filter because I wanted to avoid the amount of debris I got yesterday, this should still catch the eggs. There was a huge amount of eggs filtered:

- The tube/vial seemed to mostly be eggs and not debris

- Which of course made a large egg pellet, this is the largest egg pellet I’ve ever gotten
- Flasks were left to settle for a few hours and then imaged:
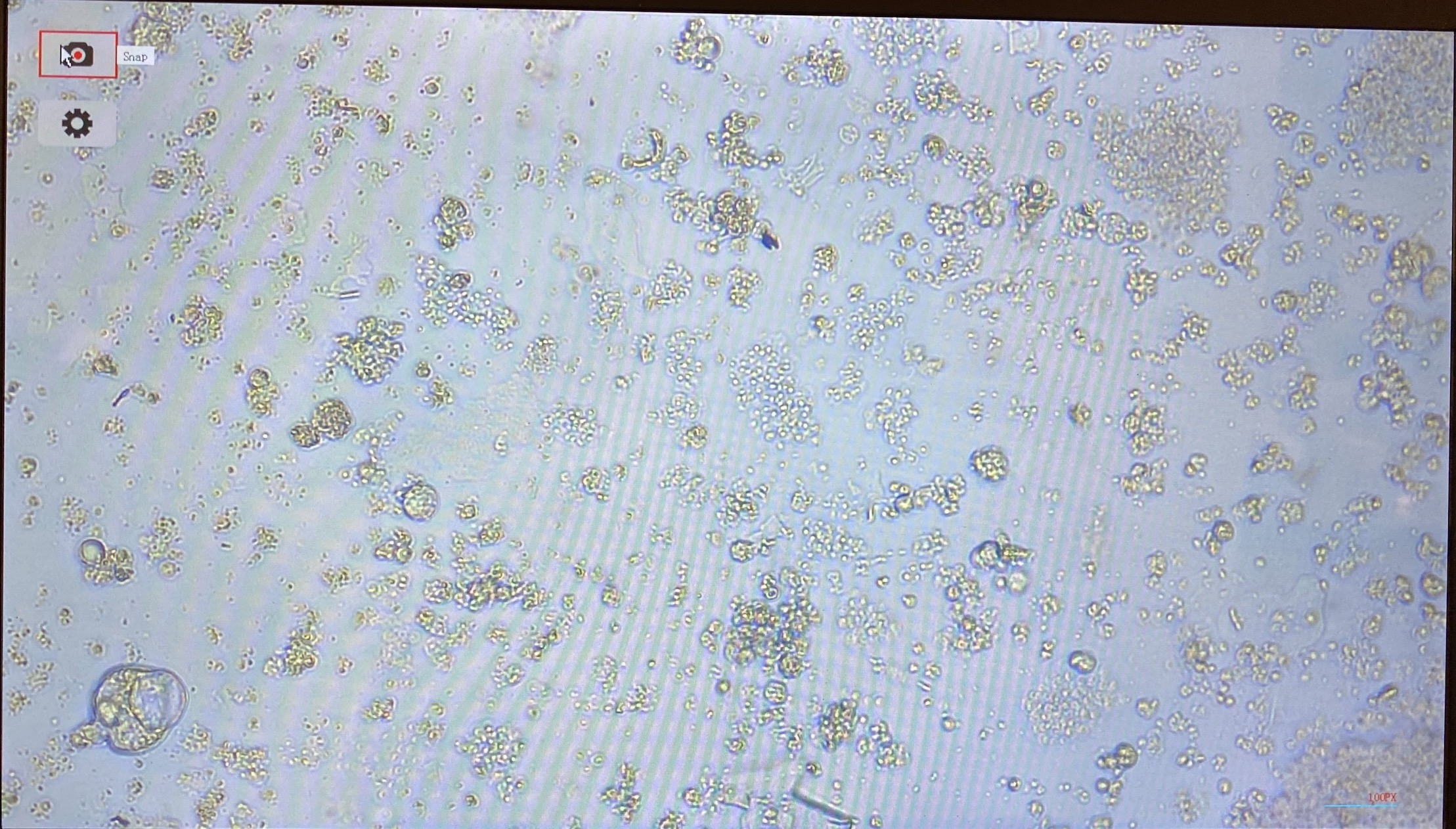
- Flasks from 20211216 had very few cells and a lot of debris
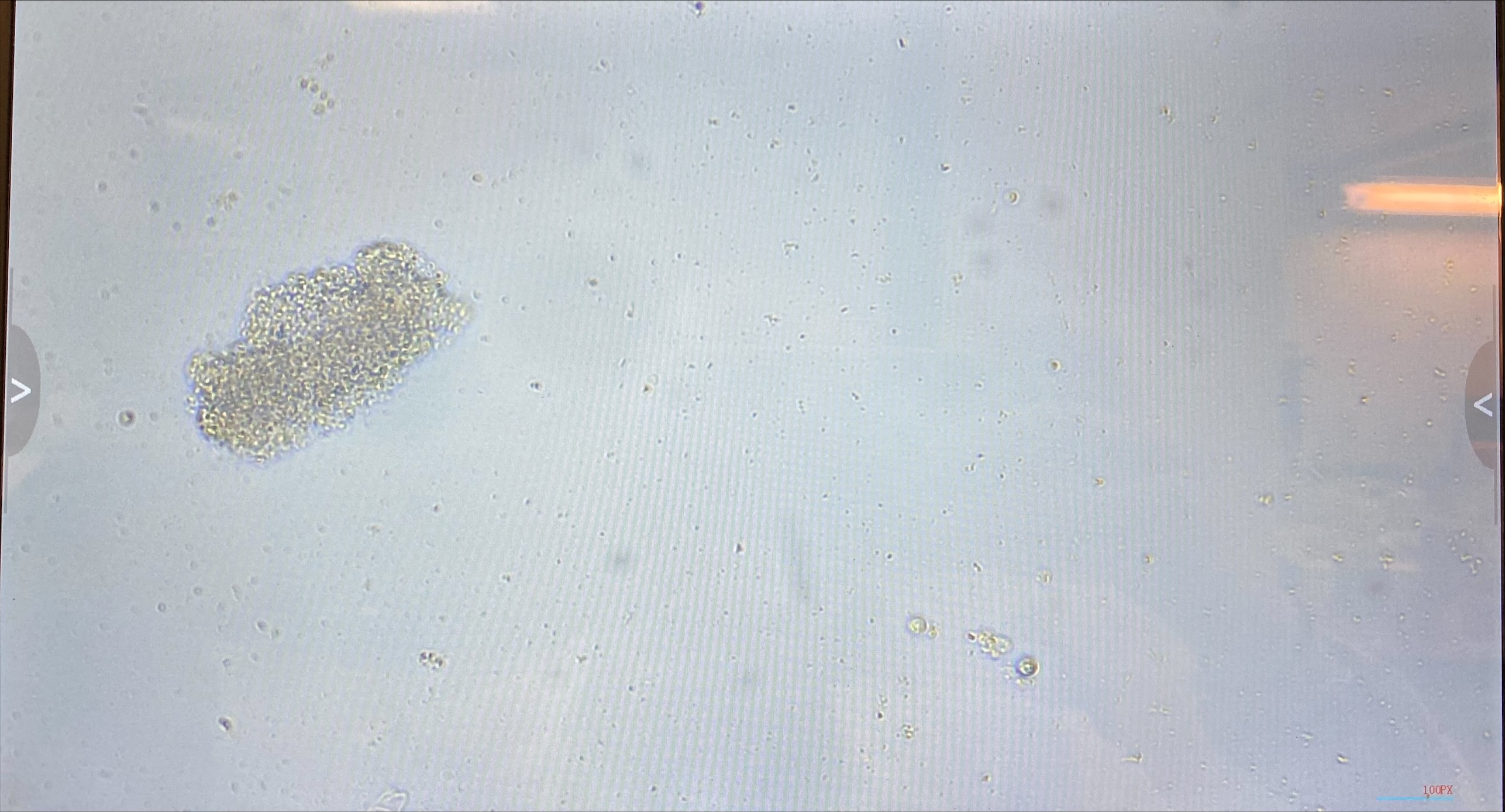
- Flasks from 20211217 had very many cells
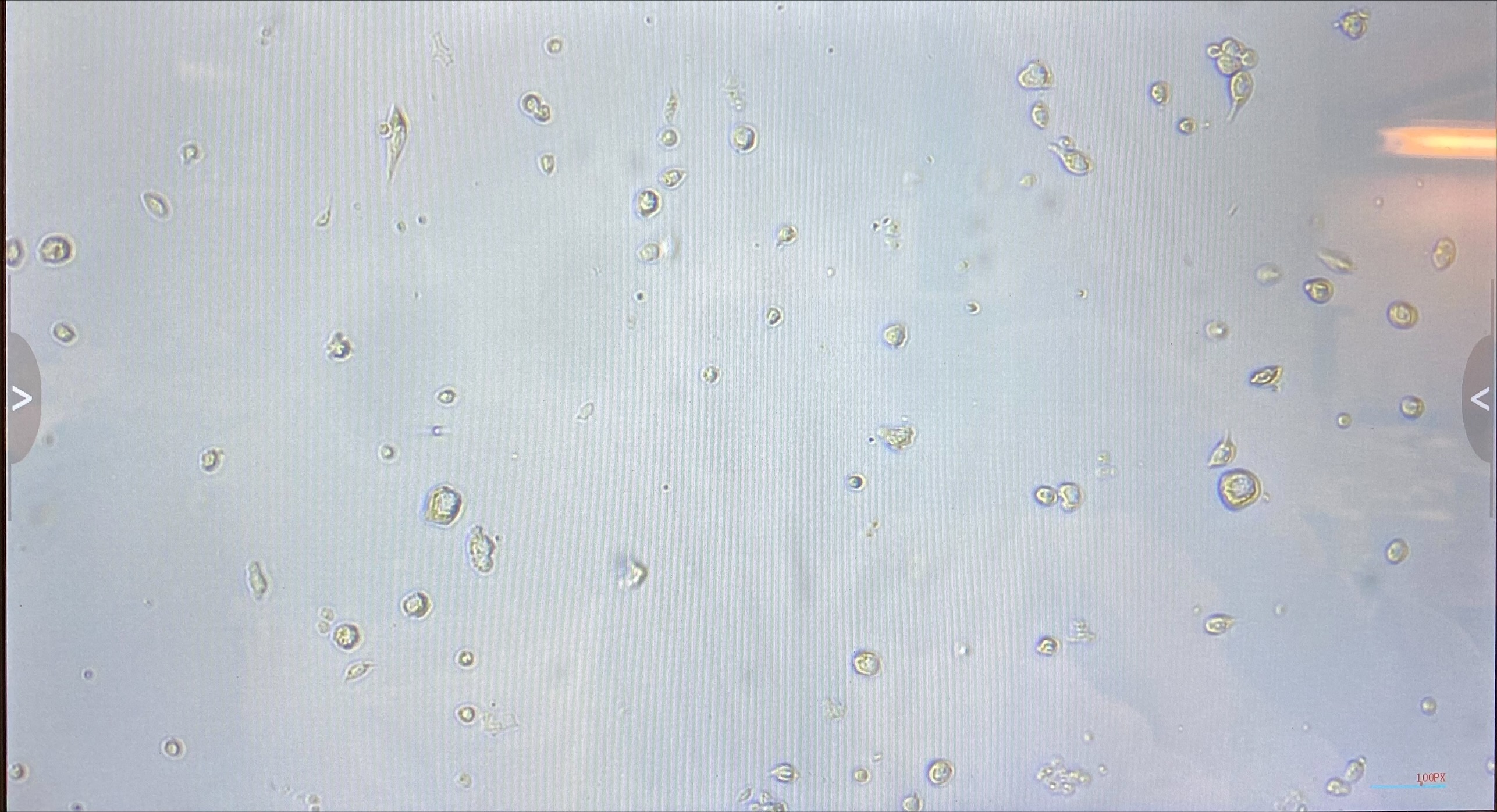
Fluid Additions and Imaging
- 1mL of 20% Schneider’s medium was added to 1 flask from 20211217. These are marked with FA
- On 20220111 an FA flask and a non FA flask were imaged. They looked similar. There is a lot of debris, most likely junk from dying cells. These cells were pelleted at an incorrect centrifugation speed so this may be why they are unhappy/dying. However it does show that there are some cells that look alive still in the flasks
- Non FA flask:
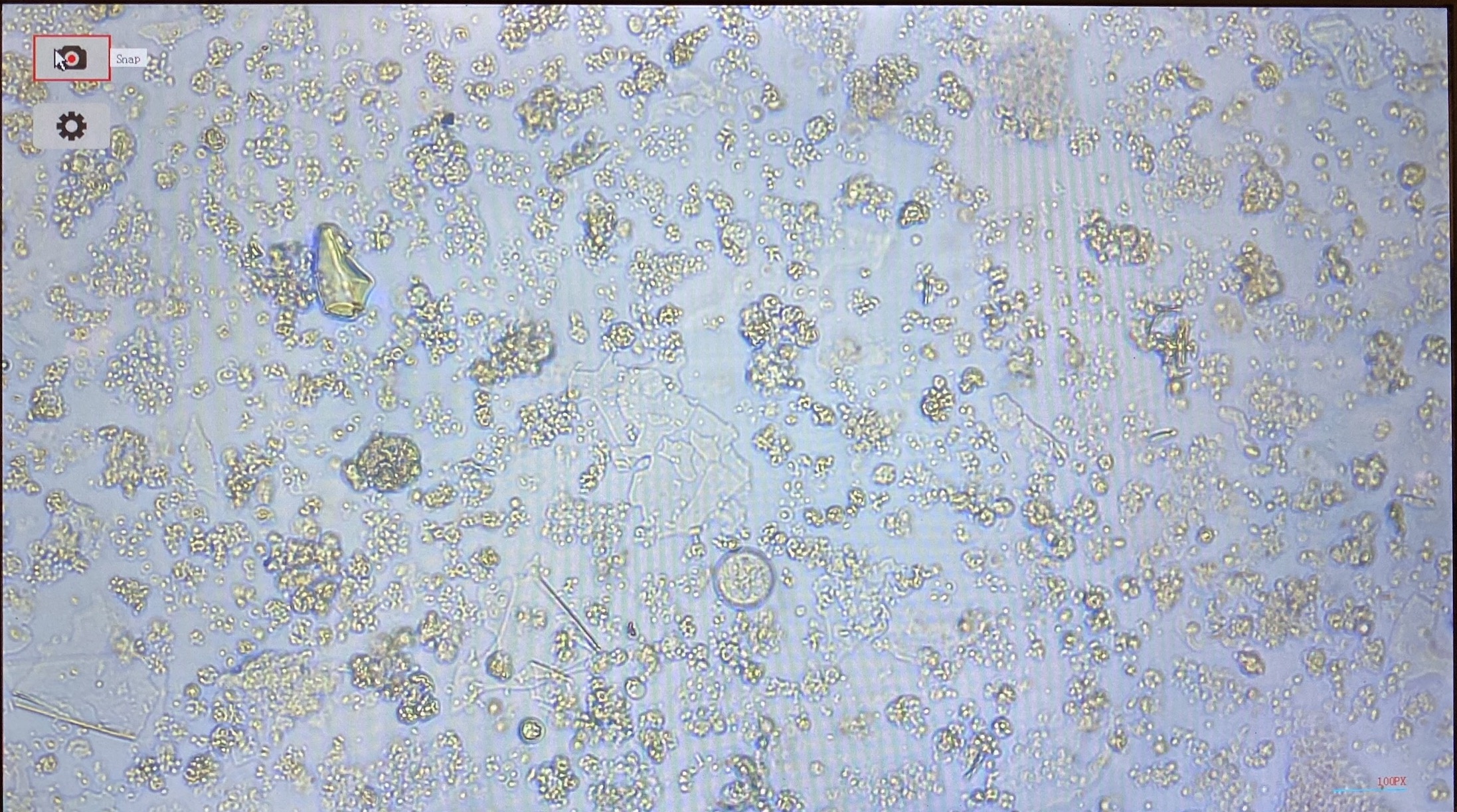
- FA flask: