3mL Input into the Genomic Tip Blood & Cell Culture DNA Midi Kit HMW Extraction Kit on a 0hr and at 120hr DiNV Infected Dv-1 Cells from Kent
Notes
- Sample Info: Kent inoculated Dv-1 cells for me last week, and froze the flask after 5 days/120 hours (should have a lot of virus at this timepoint). He also froze a flask at hour 0 that I can use as a control/extraction control/cell control/something (I’m not sure what). Unfortunately the flasks were frozen, but ideally I would have liked to have taken the cells fresh, used a cell scraper to get the cells off the bottom of the flasks, then aliquot them into single use tubes. I did that anyways, just with froze-thawed cells. I set aside 3mL for a mega extraction attempt in 15mL tubes that I kept in the fridge overnight for use the next day. The remaining 5mL I split up into 5 1.5mL tubes and stored them in the -80. For all pipetting I used a cut pipette tip.
- Samples for this extraction:
- 120hr: Dv-1 120hr inc-11-2-21 frozen 11-7-21
- 0hr: Dv-1 0hr inc-11-2-21 frozen 11-2-21
- Let the pellet dry for at least 30 min
- Going to leave the samples resuspending for over 24 hours
- I went back to the swing bucket rotor that does 4816g because I haven’t done a full extraction at that speed, and because I couldn’t find Paul in the Slusky lab to ask where the fixed angle rotor was. At least this time the pellet will be at the bottom of the tube
20211109
Sample Washing
- Placed 1X PBS and molec grade water on ice
- Kept samples on ice
- I noticed just how much more “stuff” there was in the 120hr sample (120hr on the left). I’m guessing this is because these cells had 5 more days to grow than the 0hr did

- I noticed just how much more “stuff” there was in the 120hr sample (120hr on the left). I’m guessing this is because these cells had 5 more days to grow than the 0hr did
- Set centrifuge on the 5th floor to go to 4 degrees
- Added 7mL cold PBS to each tube with 3mL sample
- Centrifuged tubes at 1,500rcf for 10 minutes at 4 degrees C
- Removed supernatant from each tube without disturbing the pellet
- There was a muchlarger pellet for the 120hr
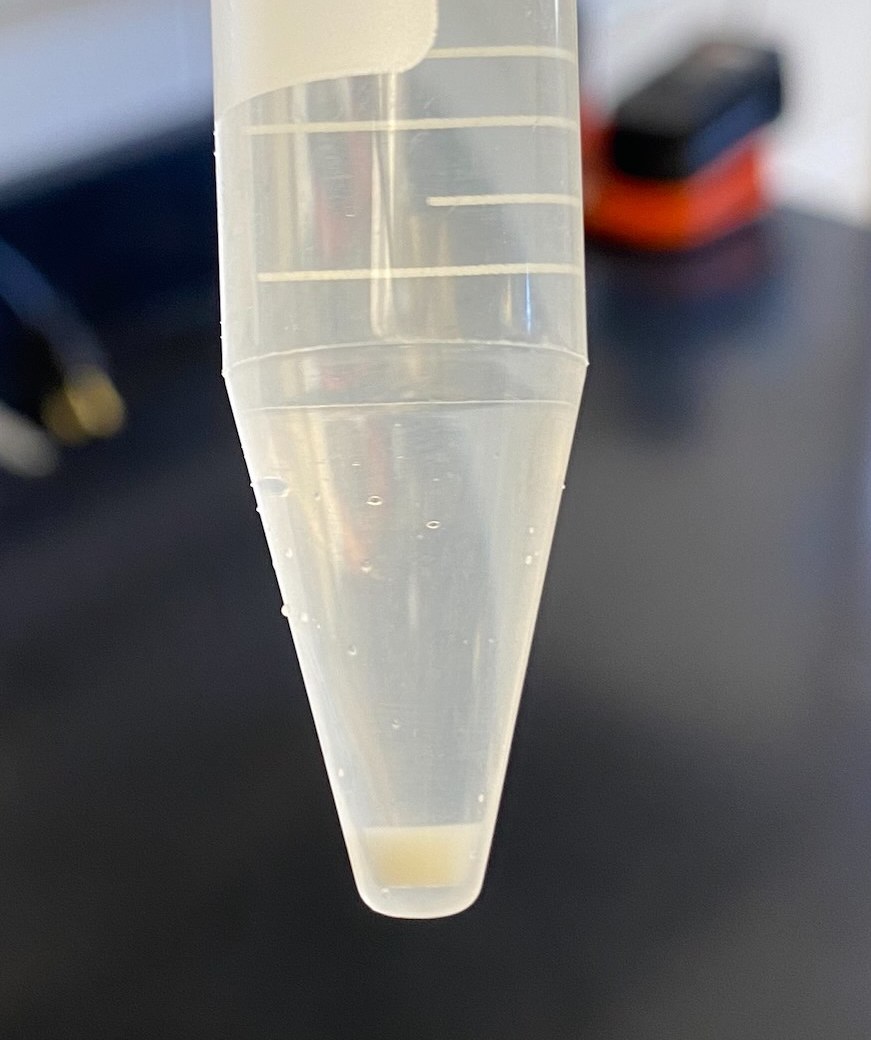
- Than for the 120hr
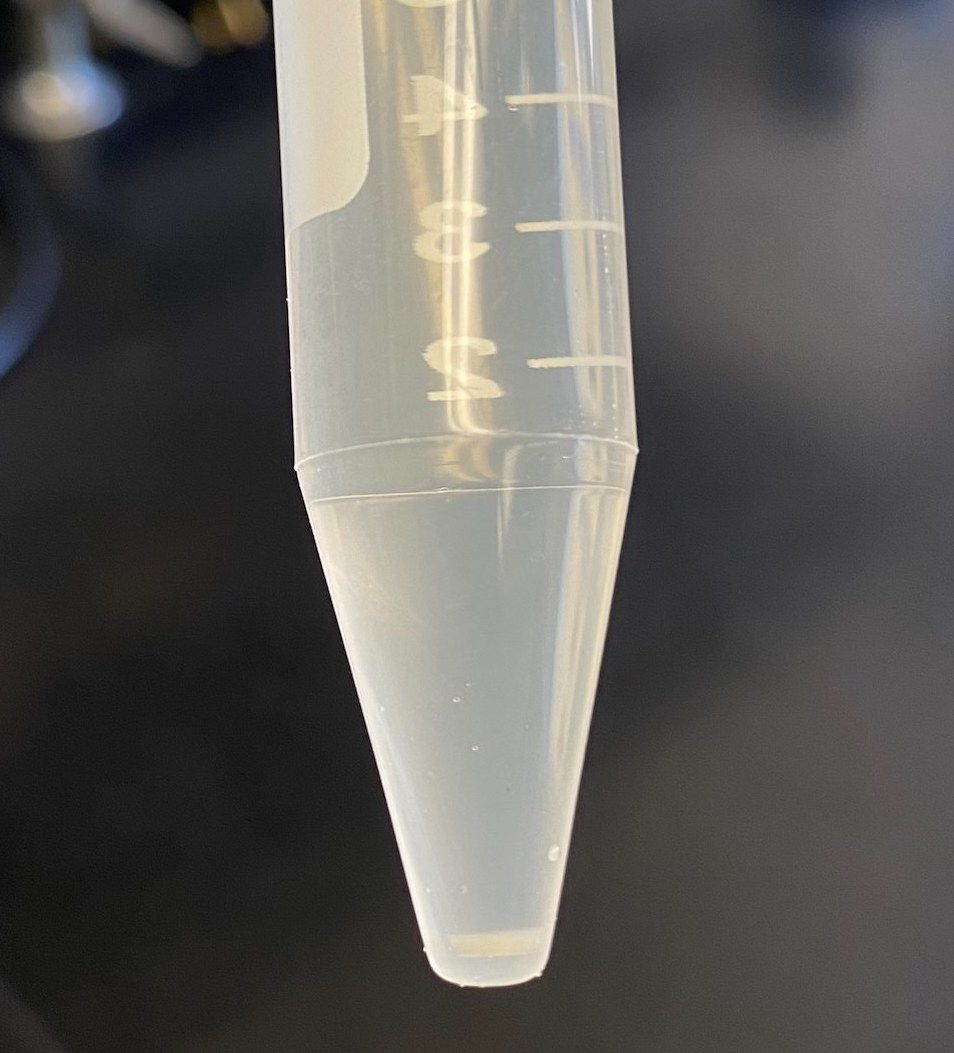
- There was a muchlarger pellet for the 120hr
- Added 10mL cold PBS to each tube
- Centrifuged tubes at 1,500rcf for 10 minutes at 4 degrees C
- Removed supernatant
- Added 2mL cold PBS to each tube
- Added 2mL cold buffer C1 to each tube
- Added 6mL cold molecular grade water to each tube
- Vortexed to get the pellet to resuspend
- Let tubes sit on ice for 10 minutes
- Centrifuged 1,300rcf for 15 minutes at 4 degrees C
- Removed supernatant without disturbing the pellet
- There was a small pellet in each sample
- Added 1mL cold buffer C1 to each tube
- Added 3mL cold molecular grade water to each tube
- Vortexed breifly
- Centrifuged 1,300rcf for 15 minutes at 4 degrees C
- Removed supernatant
Incubation
- Added 5mL buffer G2 to each tube
- Added 95mL proteinase K to each tube
- Vortexed tubes briefly
- I had to vortex these a lot, especially the 120hr because the pellet wouldn’t break up
- Placed tubes in the incubator at 50 degrees C for ~60 minutes
Genomic Tip Extraction
- Set up two tips over 50mL conicals
- Added 4mL buffer GBT to each tip and let drip
- Drip times were pretty consistent between the tips
- Vortexed sample tubes briefly
- Cut p1000 tips for each samples
- Added total volume (~5mL) of each sample to their respective tips and let drip
- Placed buffer QF in the incubator to warm to 50 degrees C
- Added 7.5mL of buffer QC to each tip
- Transferred tips to a new 15mL waste conical
- Added 7.5mL of buffer QC to each tip
- Transferred tips to new 15mL conicals labeled for final tubes
- Added 5mL of warmed buffer QF to each tip and let drip
Precipitation
- Added 4mL of 100% isopropanol to each elutent tube and inverted multiple times to mix (it looked like there was ~5.8mL of volume)
- Centrifuged tubes in the Slusky Lab at 4 degrees C 4,816rcf for 40 min (I increased the time here to hopefully get more DNA to precipitate)
- Afterwards it was very hard to see a pellet
- I thought I saw one in the 120hr sample, although it’s the bottom of the tube which can play tricks on your eyes. I was less sure about there being a pellet in the 0hr sample
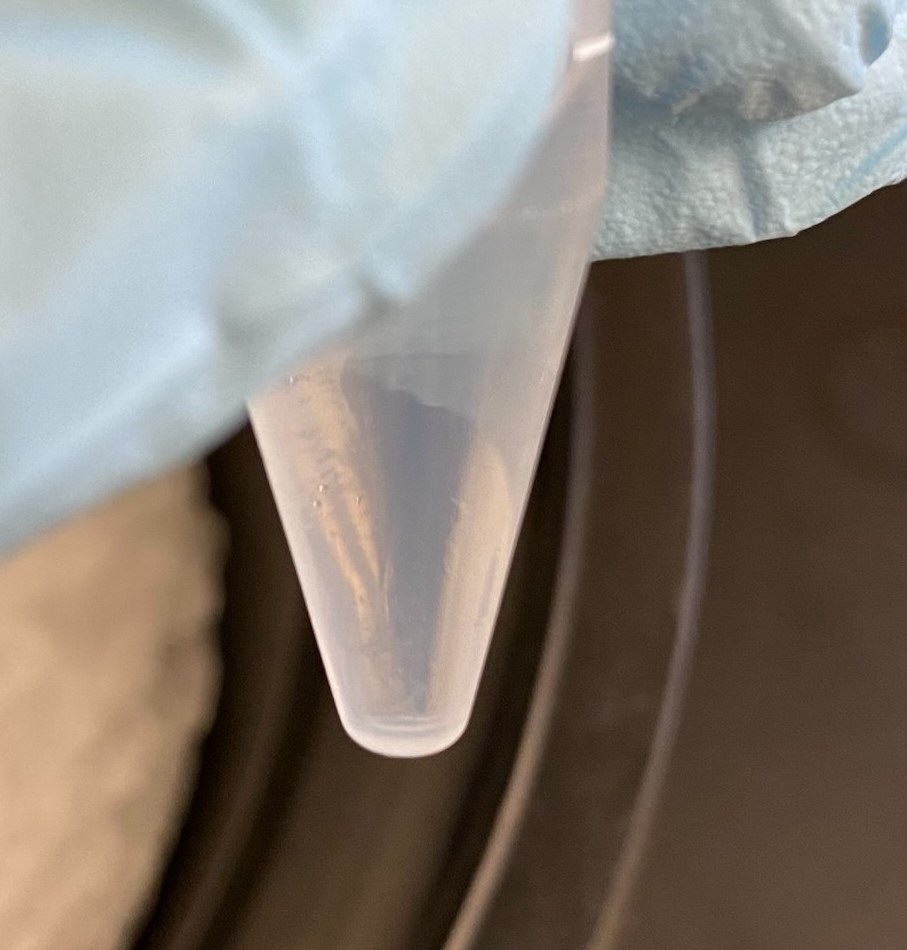
- I thought I saw one in the 120hr sample, although it’s the bottom of the tube which can play tricks on your eyes. I was less sure about there being a pellet in the 0hr sample
- Put the tubes back on ice and brought to 4055
- Removed the supernatant from each tube, pipetting from the side of the tube without the white patch to avoid any pellet
- Added 2mL cold 70% ethanol to each tube
- Vortexed tubes briefly
- Centrifuged tubes for 15 minutes at 8,816 rcf at 4 degrees C in the same centrifuge
- Tubes were transferred on ice again
- Removed the supernatant from each tube, pipetting from the side of the tube without the white patch to avoid any pellet
- Flipped the tubes upside-down on a kim wipe and let them sit for ~45 minutes to dry
- Added 50ul of 1X TE to each tube
- Tubes were placed in the incubator upright at 55 degrees C
- This incubation was supposed to be for 1 hour, but I forgot about them and only remembered after 3 hours (I came back into the lab at ~7pm). I just put the tubes on the bench for the rest of the night
20211110
- Tubes were gently flicked, spun down, and placed on a rocking stand for another 24 hrs at least
20211112
- Qubit of top and bottom of the tubes
- 0hr
- Top: 1.46ng/ul
- Bottom: 1.45ng/ul
- 120hr
- Top: 88.6ng/ul
- Bottom: 93.3ng/ul
- The 120hr is by far the best quant that I’ve gotten
- The 0hr is pretty low but I don’t think I’ll need it for anything anyways