Extracting DNA from Pentagona Sea Star with the Circulomics Nanobind HMW Kit and Using Short Read Eliminator XL
Using Nanobind Big DNA Kit with Alpha Version Reagents on another Pentagona tissue sample preserved in DNA/RNA Shield, followed mostly the recommendations of the Snail Note. Then using the Short Read Eliminator XL Kit on the DNA
Sample Prep - 20200113
- Placed dounce homogenizer, buffer CIB (same as CT), and one tube of P6 sample on ice
- While waiting for sample to thaw set the centrifuge for 4 degrees C and let run for 7 minutes to bring the temp down
- Cut two pieces of foil and sterilized forceps, got a scalpel and new blade
- Took tissue piece out of tube when thawed and cut in half on one foil sheet, minced one half with scalpel blade as much as obvious ossicles would allow
- Rest of tissue was returned to sample tube and placed back in -20 freezer
- Weighed fresh foil sheet empty and with minced tissue piece:
- Before: 0.2598g
- With tissue: 0.3105g
- Tissue weight: 0.051g or 51mg
- Goal was 60mg so this was deemed close enough
- Minced tissue was placed in the chilled dounce and 750µl of cold buffer CIB was added to the dounce
- The tissue was homogenized with the tight pestle 20 times (snail note says 15, but it looked like it needed a little more). The ossicles did not homogenize and stayed as a smushed pancake at the bottom of the dounce. This didn’t cause a problem as the tissue still moved and was broken up
- Transferred liquid and as much of the bubbles as possible to a 2mL protein lobind tube
- Centrifuged at 6000rcf for 5 minutes at 4 degrees C
- Removed supernatant without disturbing the pellet (pellet was orange) saved supernatant in case something went wrong
- Added 1mL of cold buffer CIB to the tube and pulse-vortexed 10 times
- Centrifuged at 6000rcf for 5 minutes at 4 degrees C
- Removed supernatant without disturbing the pellet saved supernatant again in case something went wrong
- Pulse-vortexed the pellet 2 times
- Added 20µl of proteinase K to the pellet
- Added 150µl of buffer CLE3 and pipette-mixed 10 times with a wide bore pipette tip
- Put tube in Thermomixer 55 degrees C 900rpm shaking for 30 minutes
- After 30 minutes checked homogenization: liquid was very opaque white but there were no visible chunks or pieces so I thought it was good to go
- Spun down in minifuge to remove liquid from cap
- Added 20µl RNase A
- Incubated in Thermomixer 55 degrees C 900rpm shaking for 30 minutes
Extraction
- Spun down in minifuge to remove liquid from cap
- Added 60µl buffer SB and pulse-vortexed 5 times sample became very white
- Centrifuged 10,000rcf for 5 minutes at room temp
- Transferred ~300µl supernatant to a new 1.5mL lobind protein tube there was a large white pellet in the tube
- Added 50µl buffer BL3 to tube with supernatant and inverted 10 times to mix liquid became cloudy with mixing
- Spun on minifuge 2 seconds
- Added 1 nanobind disk
- Added 350µl of isopropanol
- Inverted to mix 10 times became clear after mixing
- Placed on orbital mixer/shaker because it’s the only thing we have that goes 20rpm. 15 minutes on mixer at room temp
- Placed tube on magnet using method:
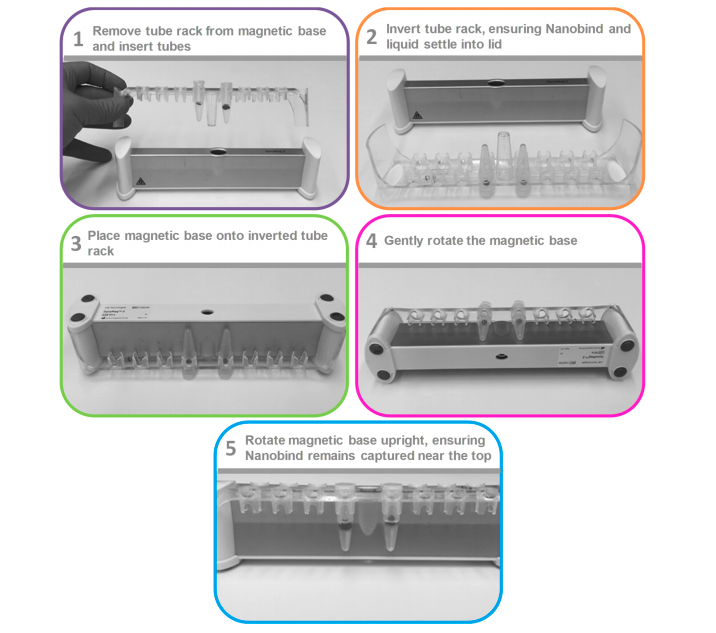
- Carefully removed and discarded supernatant
- While on magnet added 500µl CW1
- Removed tube from magnet and inverted 4 times
- Placed tube back on magnet using method
- Removed and discarded supernatant
- While on magnet added 500µl CW1
- Removed tube from magnet and inverted 4 times
- Placed tube back on magnet using method
- Removed and discarded supernatant
- While on magnet added 500µl CW2
- Removed tube from magnet and inverted 4 times
- Placed tube back on magnet using method
- Removed and discarded supernatant
- While on magnet added 500µl CW2
- Removed tube from magnet and inverted 4 times
- Placed tube back on magnet using method
- Removed and discarded supernatant
- Pipetted out residual liquid from the tube cap
- Spun down tube in minifuge
- Placed on magnet in normal way
- Removed and discarded any residual liquid
- Added 75µl buffer EB directly to the disk disk ended up being fully submerged in the buffer
- Incubated at room temp for 30 minutes
- Used wide bore tip to transfer liquid to a new 1.5mL DNA lo bind tube
- Spun down tube with disk in minifuge and pipetted up residual elutate with regular pipette tip
- Spun down tube with disk again but there was no residual liquid
- Pipetted 5 times with normal p200 pipette tip
- Left overnight at RT to let DNA solubilize
- In the morning: pipetted 5 times with normal p200 tip
BR DNA Qubit
- following protocol
- Quantified top, middle, and bottom of liquid just in case the DNA wasn’t evenly distributed
| standard 1 | standard 2 | top | middle | bottom | average |
|---|---|---|---|---|---|
| 193 RFU | 21165 RFU | 105ng/μl | 103ng/μl | 102ng/μl | 103.3ng/μl |
Genomic DNA TapeStation
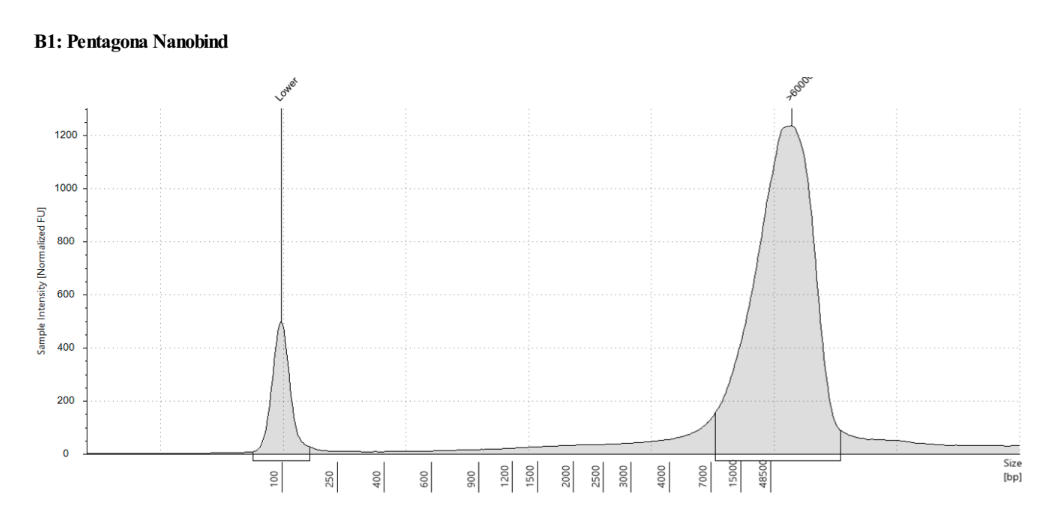
Short Read Eliminator XL Kit - 20200114
- Transferred 60µl of sample from previous extraction to a new DNA Lo bind 1.5mL tube
- Added 60µl buffer SRE XL with a wide bore tip and pipetted to mix ~7 times then tapped a bit because it didn’t look mixed
- Placed in centrifuge with tube hinge facing the outside
- Centrifuged for 30 minutes at 10,000rcf at room temp, using the non-temp controlled centrifuge
- Made fresh 80% EtOH and warmed elution buffer to 50 degrees C
- After centrifugation, removed supernatant without disturbing the pellet there was a pellet, very hard to see, looked like a small squiggle at the bottom of the tube
- Added 200µl 80% EtOH to the side of the tube without touching the pellet
- Centrifuged for 2 minutes at 10,000rcf RT
- Removed wash without disturbing the pellet
- Added 200µl 80% EtOH to the side of the tube without touching the pellet
- Centrifuged for 2 minutes at 10,000rcf RT
- Removed wash and any residual droplets
- Added 50µl warmed buffer EB and placed tube in thermomixer at 50 degrees C for 30 minutes
- Tapped tube a few times to redistributed DNA
BR DNA Qubit
- following protocol
- Quantified top, middle, and bottom of liquid just in case the DNA wasn’t evenly distributed
| standard 1 | standard 2 | top | middle | bottom | average |
|---|---|---|---|---|---|
| 200 RFU | 21694 RFU | 42.6ng/μl | 59.8ng/μl | 70.8ng/μl | 57.26ng/μl |
Genomic DNA TapeStation
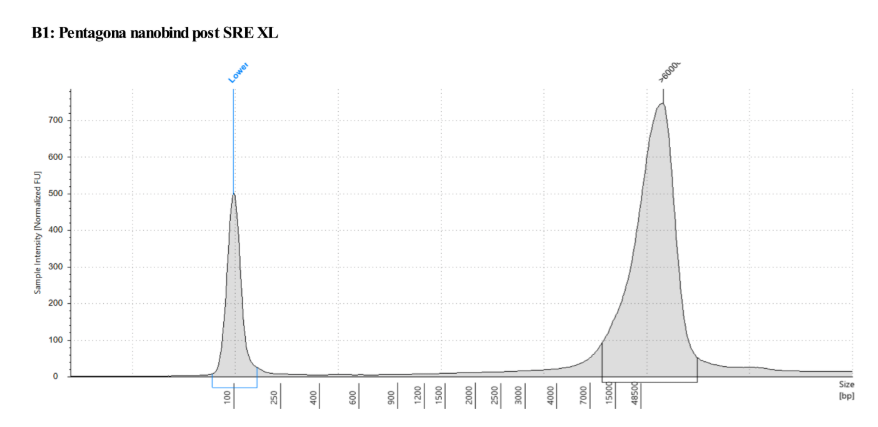
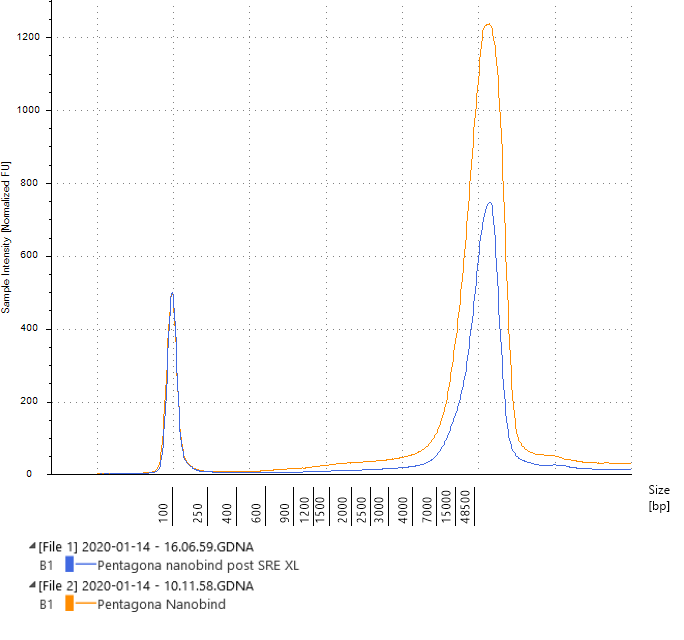
Combined of both pre and post SRE
Written on January 14, 2020